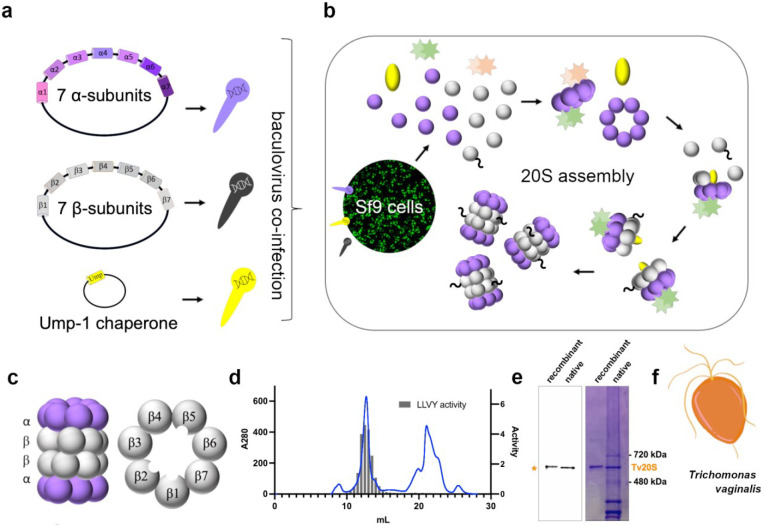
Fig. 1: Cloning, Recombinant Expression, and Purification of Tv20S Proteasome.
a) Cloning of three vectors containing either 7 α-subunits, 7 β-subunits or Ump-1 into baculovirus. b) Co-infection of SF9 cells with baculovirus for proteasome expression and assembly. c) Side view structure of the 20S proteasome complex showing two β rings sandwiched between two α rings. Planer view of the β ring showing the three catalytic subunits located within the central tunnel of the proteasome. d) Final purification step using Superose 6 chromatography. Absorbance at 280 nm (blue line) and proteasome activity assessed using a proteasome-specific fluorogenic substrate Suc-LLVY-amc (grey bar chart). MV151 and Coomassie blue-stained native PAGE showing the purity of the recombinantly purified proteasome complex compared to native Tv20S isolated from T. vaginalis. F) Cartoon representation of T. vaginalis at 100x magnification.