Abstract
Background:
The CDC and ACIP recommend COVID-19 vaccination for patients with inborn errors of immunity (IEI). Not much is known about vaccine safety in IEI and whether vaccination attenuates infection severity in IEI.
Objective:
To estimate COVID-19 vaccination safety and examine effect on outcomes in patients with IEI.
Methods:
We built a secure registry database in conjunction with the United States Immunodeficiency Network to examine vaccination frequency and indicators of safety and effectiveness in IEI patients. The registry opened on January 1, 2022 and closed on August 19, 2022.
Results:
Physicians entered data on 1,245 patients from 24 countries. The most common diagnoses were antibody deficiencies (63.7%). At least 1 COVID-19 vaccine was administered to 806 patients (64.7%), and 216 patients received vaccination prior to the development of COVID-19. The most common vaccines administered were mRNA-based (84.0%). Seventeen patients were reported to seek outpatient clinic or emergency room care for a vaccine-related complication and one patient was hospitalized for symptomatic anemia. Eight hundred twenty-three patients (66.1%) experienced COVID-19 infection. Of these, 156 patients required hospitalization (19.0%), 47 required ICU care (5.7%), and 28 died (3.4%). Rates of hospitalization (9.3% versus 24.4%, p<0.001), ICU admission (2.8% versus 7.6%, p=0.013), and death (2.3% versus 4.3%, p=0.202) in patients who had COVID-19 were lower in patients who received vaccination prior to infection. In adjusted logistic regression analysis, not having at least one COVID-19 vaccine significantly increased the odds of hospitalization and ICU admission.
Conclusion:
Vaccination for COVID-19 in the IEI population appears safe and attenuates COVID-19 severity.
Keywords: immunodeficiency, immunization, viruses: respiratory diseases, outcomes
Introduction
Coronaviruses are medium-sized, enveloped, positive-stranded RNA viruses named for their crown-like appearance under the electron microscope (1). Viruses in this family played an important role in human health long before the COVID-19 pandemic, responsible for both nonspecific upper respiratory tract infections and specific viral syndromes like severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). In 2019, cases of coronavirus-induced pneumonia clustered in the Hubei Province of China. This started out as a local outbreak in Wuhan but soon spread internationally, developing into a global pandemic. The implicated virus was designated SARS-CoV-2, and the disease that virus caused was named COVID-19.
Clinically, COVID-19 infections can range from asymptomatic to life-threatening. Several factors place infected patients at higher risk for poor outcomes, including increasing age, obesity, and chronic disease (2, 3). Among the many chronic diseases that may lead to more severe infection are inborn errors of immunity (IEI), a collection of diseases which lead to immunodeficiency and immune dysregulation. The earliest survey of 94 patients with IEI observed 10% mortality and found that risk factors for severe disease in the general public also affected outcomes in patients with IEI (4). A recently published review of the literature supports that mortality among most IEI diagnosis subgroups ranges from 4–16% (5). One large collection reported that the case fatality rate could be as high as 100 times the general population among patients 0–19 years of age. Patients with disrupted type I interferon (IFN) immunity in particular have worse outcomes (6–9).
The first COVID-19 vaccines became available in 2020, and since their introduction, vaccines have proven to be critically important to decreasing both SARS-CoV-2 spread and COVID-19 disease severity. There have been obvious concerns regarding how well vaccines work in patients with IEI. Few studies have been performed, and the highly varied nature of the more than 450 IEI disorders make generalization across disorders impossible. IEI patients can have lower rates of seroconversion compared to healthy controls, though responses tend to improve with additional (third dose) vaccine administrations or when given in the setting of previous COVID-19 infection (5, 10–13). In addition to antibody responses to COVID-19 vaccination, T cell responses have proven to be important (14, 15). Pham and colleagues found that most patients with IEI were able to mount at least a T cell response to vaccination, even if their humoral immune response to vaccination was lackluster (16). These findings suggested the importance of COVID-19 vaccination even in patients with severe humoral immune defects, as these patients may benefit from adaptive cellular immune responses even in the absence of a functioning humoral immune system.
Regardless of laboratory-observed vaccination responses, an important consideration for both IEI patients and practicing immunologists is the real-world effectiveness of vaccination against SARS-CoV-2. A previous large healthcare claims study of vaccinated patients found that most infections after vaccination resulting in hospitalization and/or death occurred in patients with primary and secondary immunodeficiencies, but no data were presented regarding just primary IEI patients (17). A small single-center study of COVID-19 outcomes in 113 IEI patients who predominantly had CVID, hypogammaglobulinemia, and agammaglobulinemia observed that COVID-19 related hospitalization occurred in 40% of unvaccinated patients versus 4% in vaccinated patients, suggesting a dramatic protective effect of vaccination (18).
Overall, COVID-19 vaccines have proven safe in the general population, although mild local and systemic reactions are common such as pain, lymphadenopathy, headache, and fever. Although severe events are possible, including myocarditis, pericarditis, anaphylaxis, and thromboembolic events, these serious adverse events are rare (19, 20). Little is known about the safety of COVID-19 vaccines in patients with IEI. For example, patients with autoinflammatory IEIs may fear that vaccination could precipitate a disease flare, requiring increased immunosuppression or hospitalization. Indeed, the rarity of many IEI conditions and the relative recency of COVID-19 disease has made it difficult for professional organizations, the normal adjudicators of such questions, to be able to determine if there are unique or increased risks for these patients. In fact, the 2021 consensus statement from the European Alliance of Associations for Rheumatology and the American College of Rheumatology on diagnosis and management of type 1 interferonopathies expresses agnosticism, stating “whether vaccines against COVID-19 have the potential to provoke a disease flare is unknown” and “there are currently no data to back specific recommendations” (21).
In this study, we investigated the real-world safety and effectiveness of vaccination in IEI patients. Our findings demonstrate that COVID-19 vaccination is safe and effective in a large, phenotypically diverse, and multinational IEI registry including more than 1000 patients.
Materials and Methods
This study was performed as a collaboration between Cincinnati Children’s Hospital, the United States Immunodeficiency Network (USIDNET), the Clinical Immunology Society, and additional physicians who contributed patient data. We created a COVID-19-specific registry database as part of the USIDNET for the collection of IEI patient data related to SARS-CoV-2 infection and/or SARS-CoV-2 vaccination. This REDCap database was used to house de-identified clinical patient data submitted by immunologists worldwide. The study was approved as exempt research by the Cincinnati Children’s Hospital institutional review board (IRB ID: 2021 – 0406). Members of the Clinical Immunology Society (CIS) were invited by email to contribute patient data via entry into the registry database. The database opened for entries on January 1, 2022 and closed on August 19, 2022.
Patients of any age with IEI and COVID-19 infection, COVID-19 vaccination, or both were eligible for inclusion. Diagnoses were linked to International Union of Immunological Societies (IUIS) subcategories by phenotypic or molecular defect, wherever possible (22). All types of COVID-19 infections and complications were eligible for inclusion – asymptomatic, acute, long COVID, and multisystem inflammatory syndrome in children or adults (MIS-C/MIS-A). In addition to basic demographics and information on the nature of the subject’s IEI and relevant medical comorbidities, data were collected on hospitalization, requirement of ICU care, and patient survival. For patients who had COVID-19 vaccination, data were collected on vaccine side effects, need for escalation of IEI treatment in relation to vaccination, and health care utilization.
Primary outcomes of interest were 1) adverse vaccine effects and 2) real-world vaccine effectiveness in preventing hospitalization, ICU admission, and death. Adverse vaccine effects were determined by analyzing reported need for medical care in association with vaccination (emergency, outpatient, and inpatient environments), evaluating for significant changes to patients’ immunology medication regimen, examining for development of vaccine-induced myocarditis and anaphylaxis, and reviewing reported adverse effects beyond expected pain and fever for up to 3 days. Real-world vaccine effectiveness was assessed by comparison of reported hospital admission, ICU admission, and death in patients with at least one vaccine dose versus those without. Consideration of the timing of a COVID-19 infection in relation to vaccination was built into the analysis. For example, subjects who had a COVID-19 infection prior to vaccination were analyzed differently from subjects with first infection after vaccination.
We report descriptive statistics for the study population, including medians for continuous variables and counts and percentages for categorical variables. Categorical variables were compared using the Pearson chi-square test. Logistic regression analysis for outcomes of non-ICU hospitalization, ICU hospitalization, and death against vaccination status were performed both unadjusted and adjusted for potential confounding factors. Confounders considered in the adjusted models were age, obesity, kidney disease, lung disease, immunosuppressive medication use in the previous three months, neuromuscular disease, tracheostomy status, heart disease, sickle cell disease, and diabetes. Confounders that occurred infrequently in the data set (< 20 times each in the entire cohort) – namely, neuromuscular disease, tracheostomy, heart disease, diabetes, and sickle cell disease – were grouped together into a composite risk factor binary variable. For all these confounders, we assumed nonresponse on the survey instrument to be equivalent to a “no” answer, as not all fields were required for submission of a patient entry. All analysis was performed using Stata 17.0 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC).
Results
Patients
A total of 1,245 subjects were entered in the registry (TABLE 1). Of these, 806 (64.7%) had received at least one vaccine against COVID-19. The type of vaccine received was reported for 80.7% of vaccinated patients: the majority of patients received mRNA-based vaccines (84.0%) followed by viral vector (10.9%) and protein subunit vaccines (5.1%). Seven-hundred twenty-five received 2 or more vaccinations. Males were slightly predominant (53%), and patients were mostly Caucasian (82.4%) and from the U.S.A. (63.5%). Demographic characteristics were generally similar between the unvaccinated and vaccinated groups. Major exceptions were age (vaccinated patients tended to be older) and country of the treating medical center (higher percentage of vaccinated patients in U.S. centers). The burden of comorbidities including lung disease, obesity, diabetes, and other conditions was similar between the groups, although the proportion of patients with history of bone marrow transplant (n = 96) was higher in the vaccinated group (8.9% compared to 5.5%).
TABLE 1.
Demographic characteristics of subjects in the USIDNET Registry.
| Overall | ≥1 Vaccine | No Vaccine | |
|---|---|---|---|
| Demographics | 1,245 | 806 (64.7) | 439 (35.3) |
| Sex | |||
| Female | 585 (47.0) | 379 (47.0) | 206 (46.9) |
| Male | 660 (53.0) | 427 (53.0) | 233 (53.1) |
| Race/Ethnicity* | |||
| White | 873 (70.1) | 567 (70.3) | 306 (69.7) |
| Black | 57 (4.6) | 34 (4.2) | 23 (5.2) |
| Native American | 3 (0.2) | 3 (0.4) | 0 (0.0) |
| Asian | 31 (2.5) | 19 (2.4) | 12 (2.7) |
| Hawaiian | 5 (0.4) | 3 (0.4) | 2 (0.5) |
| Other | 91 (7.3) | 52 (6.4) | 39 (8.9) |
| Not Reported | 185 (14.9) | 128 (15.9) | 57 (13.0) |
| Age at entry (years) median (p25-p75) (min-max)* | 22 (14–43) (0–80) | 28 (17–48) (4–80) | 15 (8–26) (0–80) |
| Evaluating center | |||
| US-based* | 791 (63.5) | 529 (65.6) | 262 (59.7) |
| Comorbidities | |||
| Lung disease^ | 440 (35.3) | 290 (36.0) | 150 (34.2) |
| Obesity^ | 154 (12.4) | 107 (13.3) | 47 (10.7) |
| Neuromuscular disease^ | 3 (0.4) | 3 (0.4) | 0 (0) |
| Tracheostomy^ | 6 (0.5) | 3 (0.4) | 3 (0.7) |
| Heart disease^ | 16 (1.3) | 11 (1.4) | 5 (1.1) |
| Sickle cell disease^ | 1 (0.1) | 1 (0.1) | 0 (0) |
| Diabetes^ | 10 (0.8) | 7 (0.9) | 3 (0.7) |
| Renal disease^ | 67 (5.4) | 44 (5.5) | 23 (5.2) |
| Immunosuppressive medications (past 3 months)^^ | 238 (19.1) | 165 (20.5) | 73 (16.6) |
| Bone marrow transplant history^ | 96 (7.7) | 72 (8.9) | 24 (5.5) |
Data are presented as no. (%) unless otherwise indicated
variable had missing data
includes assumed variables - if entry was left blank assumed response was “no”
immunosuppressive medication as determined by physician entering data for patient
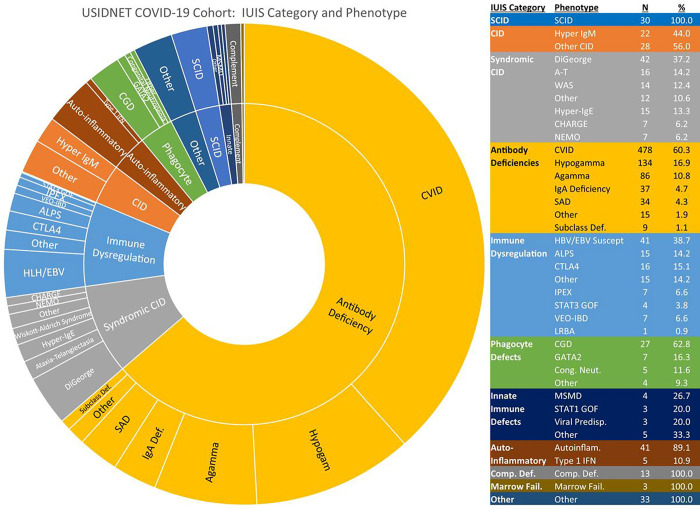
A wide variety of IEI phenotypes and molecular diagnoses were represented in the cohort (FIGURE 1). Most patients (n = 793) had antibody defects, predominantly CVID, hypogammaglobulinemia, and agammaglobulinemia. Combined immune deficiencies, syndrome-associated and otherwise, together made up the second largest category (n = 163). Disorders of immune dysregulation, including primary immune regulatory disorders and genetic disorders associated with hemophagocytic lymphohistiocytosis (HLH) or Epstein-Barr virus (EBV)-susceptibility, were also well represented (n = 106). Forty-six patients had auto-inflammatory disorders. There were 27 patients with chronic granulomatous disease (CGD) and 16 patients with other disorders of phagocyte function or number. Remaining categories are shown in FIGURE 1. Note that 33 patients did not have enough information recorded to be categorized into a specific grouping. The breakdown of molecular diagnoses is given in Table 2 and included close to 150 different genetic diagnoses. The genetic disorders in the registry with 10 or more patients reported included pathogenic changes in ADA, CD40L, ATM, WAS, BTK, TNFRSF13B, CTLA4, XIAP, and 22q11 deletion.
Figure 1.
Patient diagnoses in USIDNET Registry, categorized by International Union of Immunologic Societies (IUIS) schema. General IUIS categories further subclassified based on phenotype or genetic defect.
Abbreviations: SCID, severe combined immune deficiency; CID, combined immune deficiency; A-T, ataxia-telangiectasia; WAS, Wiskott-Aldrich syndrome; CHARGE, coloboma/heart defects/atresia choanae/growth retardation/genital abnormalities/ear abnormalities; NEMO, nuclear factor-kappa B essential modulator deficiency; CVID, common variable immune deficiency; hypogamma, hypogammaglobulinemia; agamma, agammaglobulinemia; Comp. Def., complement deficiency; SAD, specific antibody deficiency; Subclass Def., IgG subclass deficiency; IgA Def., IgA Deficiency; HLH/EBV Susc., hemophagocytic lymphohistiocytosis and EBV susceptibility; ALPS, autoimmune lymphoproliferative syndrome; IPEX, immune dysregulation/polyendocrinopathy/enteropathy/X-linked syndrome; VEO-IBD, very early onset inflammatory bowel disease; CGD, chronic granulomatous disease; MSMD, Mendelian susceptibility to mycobacterial disease; Cong. Neut., congenital neutropenia; Marrow Fail., bone marrow failure; Viral Predisp., predisposition to severe viral infection
Table 2.
Molecular defects of subjects in USIDNET Registry.
| Category | Defect | N (%) | Category | Defect | N (%) | Category | Defect | N (%) | Category | Defect | N (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SCID | ADA | 12 (40.0) | (Antibody | NFKB1 | 8 (1.0) | Immune | nr | 38 (35.9) | Autoinflammatory | NLRP3 | 6 (13.0) |
| nr | 5 (16.7) | Def. Cont) | NFKB2 | 7 (0.9) | Dysregulation | CTLA4 | 16 (15.1) | ADA2 | 5 (10.9) | ||
| RAG1 | 3 (10.0) | STAT3 GOF | 4 (0.5) | XIAP | 11 (10.4) | MEFV | 4 (8.7) | ||||
| IL7R | 2 (6.7) | 47 + 21 | 3 (0.4) | FOXP3 | 5 (4.7) | MVK | 4 (8.7) | ||||
| LIG4 | 2 (6.7) | CTLA4 | 3 (0.4) | AIRE - AR | 4 (3.8) | IL1RN | 3 (6.5) | ||||
| DCLRE1C | 1 (3.3) | IGLL1 | 3 (0.4) | SH2D1A | 4 (3.8) | PSTPIP1 | 3 (6.5) | ||||
| IL2RA | 1 (3.3) | Kabuki | 3 (0.4) | STAT3 GOF | 4 (3.8) | nr | 3 (6.5) | ||||
| IL2RG | 1 (3.3) | NOD2 | 3 (0.4) | STXBP2 | 3 (2.8) | CARD14 | 2 (4.4) | ||||
| JAK3 | 1 (3.3) | 16p11 | 2 (0.3) | TNFRSF6 | 3 (2.8) | CDC42 | 2 (4.4) | ||||
| NHEJ1 | 1 (3.3) | DUOX2 | 2 (0.3) | UNC13D | 3 (2.8) | TMEM173 | 2 (4.4) | ||||
| RAG2 | 1 (3.3) | IKBKB | 2 (0.3) | FAS | 2 (1.9) | ATP6AP1 | 1 (2.2) | ||||
| CID | nr | 20 (40.0) | IKZF1 | 2 (0.3) | LRBA | 2 (1.9) | COPA | 1 (2.2) | |||
| CD40L | 12 (24.0) | IRF2BP2 | 2 (0.3) | AIRE - AD | 1 (0.9) | NFKB1 | 1 (2.2) | ||||
| AICDA | 6 (12.0) | LRBA | 2 (0.3) | ELF4 | 1 (0.9) | IFIH1 | 1 (2.2) | ||||
| RAG1 | 2 (4.0) | PIK3R1 - AD | 2 (0.3) | IPEX | 1 (0.9) | S124F | 1 (2.2) | ||||
| CARD11 | 1 (2.0) | TCF3 | 2 (0.3) | LYST | 1 (0.9) | NLRC4 | 1 (2.2) | ||||
| CARMIL2 | 1 (2.0) | 47 XXY | 1 (0.1) | MAGT1 | 1 (0.9) | PEPD | 1 (2.2) | ||||
| CD70 | 1 (2.0) | ADA2 | 1 (0.1) | PRKCD | 1 (0.9) | RELA | 1 (2.2) | ||||
| IL2RA | 1 (2.0) | ATM | 1 (0.1) | RAB27A | 1 (0.9) | TNFAIP3 | 1 (2.2) | ||||
| PGM3 | 1 (2.0) | BLNK | 1 (0.1) | SLC7A7 | 1 (0.9) | TNFRSF1A | 1 (2.2) | ||||
| PIK3R1 | 1 (2.0) | C1QA | 1 (0.1) | STX11 | 1 (0.9) | TNFSF13 | 1 (2.2) | ||||
| RMRP | 1 (2.0) | CARD11 | 1 (0.1) | TNFAIP3 | 1 (0.9) | TRNT1 | 1 (2.2) | ||||
| SASH3 | 1 (2.0) | CD21 | 1 (0.1) | TPP2 | 1 (0.9) | Complement | nr | 8 (61.5) | |||
| STK4 | 1 (2.0) | CD40 | 1 (0.1) | Phagocyte | nr | 21 (48.8) | C1S | 1 (7.7) | |||
| TNFRSF13B | 1 (2.0) | CXCR4 | 1 (0.1) | GATA2 | 7 (16.3) | C2 | 1 (7.7) | ||||
| Syndromic | 22q11 | 40 (35.4) | DNASE2 | 1 (0.1) | CYBB | 6 (14.0) | C4A + C4B | 1 (7.7) | |||
| CID | ATM | 16 (14.2) | DNMT3B | 1 (0.1) | NCF1 | 4 (9.3) | C5 | 1 (7.7) | |||
| WAS | 14 (12.4) | DOCK2 | 1 (0.1) | CYBA | 1 (2.3) | CFI | 1 (7.7) | ||||
| STAT3 LOF | 13 (11.5) | ERCC2 | 1 (0.1) | FCGR3A | 1 (2.3) | Unknown (n = 33) | |||||
| CHD7 | 7 (6.2) | FANCA | 1 (0.1) | HAX1 | 1 (2.3) | ||||||
| IKBKG (NEMO) | 7 (6.2) | GATA2 | 1 (0.1) | JAGN1 | 1 (2.3) | nr = not reported | |||||
| nr | 5 (4.4) | HYOU1 | 1 (0.1) | NCF4 | 1 (2.3) | ||||||
| KMT2D | 4 (3.5) | IGHM | 1 (0.1) | Innate | IL17RA | 3 (20.0) | |||||
| TBX1 | 2 (1.8) | IRF7 | 1 (0.1) | STAT1 GOF | 3 (20.0) | ||||||
| FOXI3 | 1 (0.9) | LIG4 | 1 (0.1) | IL12RB1 | 2 (13.3) | ||||||
| KMT2A | 1 (0.9) | NCKAPIL | 1 (0.1) | MYD88 | 2 (13.3) | ||||||
| NFKBIA | 1 (0.9) | PIK3CG | 1 (0.1) | IFNAR1 | 1 (6.7) | ||||||
| RMRP | 1 (0.9) | PIK3R1 - AR | 1 (0.1) | IFNGR1 - AD | 1 (6.7) | ||||||
| SKIV2L | 1 (0.9) | PLCG2 | 1 (0.1) | IFNGR1 - AR | 1 (6.7) | ||||||
| Antibody | nr | 633 (79.8) | POLG | 1 (0.1) | TLR3 | 1 (6.7) | |||||
| Def. | BTK | 56 (7.1) | TCIRG1 | 1 (0.1) | nr | 1 (6.7) | |||||
| TNFRSF13B | 17 (2.1) | TRNT1 | 1 (0.1) | Bone Marrow | DKC1 | 1 (33.3) | |||||
| PIK3CD | 9 (1.1) | TOP2B | 1 (0.1) | Failure | SAMD9 | 1 (33.3) | |||||
| WDR19 | 1 (0.1) | nr | 1 (33.3) |
Vaccine Complications
Adverse events reported after vaccination are listed in Table 3. Of the 806 patients who received at least one vaccine, only 17 were reported to seek medical care in the outpatient clinic (n = 9) or emergency room (n = 8) for a vaccine-related complication. One patient was hospitalized in association with the COVID-19 vaccine. This patient had a diagnosis of hyper-IgM syndrome and a history of recurrent cytopenias and developed symptomatic anemia after his first COVID-19 vaccine.
Table 3.
Vaccine safety in the IEI Cohort (n = 806).
| Emergency Room Visit | 8 (1.0) | Significant treatment changes | 9 (1.2) | |
| Outpatient Clinic Visit | 9 (1.1) | Increased immunosuppression | 4 (0.5) | |
| IPEX patient with worsening eczema, required topical corticosteroids and dupilumab | ||||
| Hospitalization | 1 (0.1) | STAT1 GOF patient developed severe aphthous ulcers, required baricitinib | ||
| Specific antibody deficiency patient with asthma exacerbation requiring prednisone | ||||
| Adverse Effects / Frequency (Some patients with > 1) | 25 (3.1) | XIAP patient took previously prescribed oral steroids for possible disease flare | ||
| fatigue | 8 | Increased infections/requirement for antibiotics | 3 (0.4) | |
| myalgia | 5 | CVID patient, developed herpetic infections, required antiviral | ||
| arthralgia | 3 | MSMD patient developed Salmonella bacteremia and lymphadenitis | ||
| headache | 3 | CVID patient started on antibiotic prophylaxis, unclear indication | ||
| chest pain | 2 | |||
| congestion | 2 | Other | 2 (0.3) | |
| diarrhea | 2 | Hereditary angioedema patient had increased disease flares requiring increased C1 INH | ||
| palpitations | 2 | CVID patient developed cholelithiasis requiring surgery, 4 months after vaccine | ||
| abdominal pain | 1 | |||
| confusion | 1 | Anaphylaxis | 0 (0) | |
| costochondritis | 1 | |||
| cough | 1 | Myocarditis | 0 (0) | |
| diabetes | 1 | |||
| fever (> 3 days) | 1 | MIS-C/MIS-A | 1 (0.1) | |
| flushing | 1 | |||
| headache | 1 | |||
| lethargy | 1 | |||
| lymph node pain | 1 | |||
| malaise | 1 | |||
| nausea | 1 | |||
| rash | 1 | |||
| serum sickness | 1 | |||
| sore throat | 1 | |||
| unspecified | 1 | |||
Twenty-five patients were reported as having adverse effects secondary to the vaccines, with several having more than one listed complaint. Common issues included fatigue (8 patients), myalgia (5 patients), arthralgia (3 patients), and headache (3 patients). Most adverse effects were mild and self-limited, although one patient, a teenage girl with CVID, developed a serum sickness-like reaction which required an outpatient physician visit and treatment with oral antihistamines. Seven patients required either increased immunosuppression or a change in antibiotic regimen after vaccination as detailed in the table. It is not clear what role vaccination may have played in many of these events, and some (e.g. onset of diabetes, gallstones) seem likely unrelated. There were no cases of anaphylaxis or vaccine-related myocarditis. MIS-C/MIS-A was reported to occur in 1 patient following vaccination. This patient had his second COVID vaccination, followed by a mild COVID-19 infection six days later. He then developed MIS-C approximately 6 weeks after the infection and required ICU care. Ultimately, this patient was diagnosed with familial HLH due to pathogenic variants in STXBP2.
COVID-19 Infection
Sixty-six percent (n = 823) of the patients in the USIDNET cohort experienced COVID-19 infections (Table 4). Of these, the majority (89.2%) were acute/symptomatic. MIS-C/MIS-A were reported in 7/823 patients (0.8%). The underlying IEI diagnoses in patients with MIS-C/MIS-A were X-linked agammaglobulinemia (n = 2), Hyper-IgM syndrome (n = 2), interferonopathy (n = 1), inherited bone marrow failure (n = 1), and APECED (n = 1). Long COVID was reported in 13/823 patients (1.6%). One-hundred and fifty-one patients (18.4% of those infected) received monoclonal antibodies to prevent or treat COVID-19, and 41 (5.0% of those infected) received convalescent plasma. One-hundred and fifty-six IEI patients infected with SARS-CoV-2 required hospitalization (19.0% of those infected), 47 required ICU care (5.7%), and 28 died (3.4%) (FIGURE 2). Characteristics of the 28 patients who died are given in Table 5. Most deceased patients had multiple co-morbidities. The cause(s) of death for most adult patients included COVID-19, pneumonia, respiratory failure, acute respiratory distress syndrome (ARDS), or multi-organ failure. Sepsis was more commonly reported as an additional or only cause of death in pediatric patients, with Escherichia coli and Stenotrophomonas maltophilia identified in 2 patients.
Table 4.
COVID-19 outcomes in the IEI Cohort and effect of vaccination. Data are presented as column totals/percentages for outcomes of infection, and hospitalization, ICU admission, and death among those infected.
| IEI patient with ≥ 1 COVID-19 infection | N = 823 | P | |
|---|---|---|---|
| asymptomatic | 64 (7.8%) | ||
| acute | 734 (89.2%) | ||
| MIS-C/MIS-A | 7 (0.8%) | ||
| long | 13 (1.6%) | ||
| other | 5 (0.6%) | ||
| Required hospitalization for COVID-19 infection | N = 156 (19.0%) | P < 0.001 | |
| history of ≥ 1 vaccine prior to hospitalization | 20 (12.8%) | ||
| no vaccination prior to hospitalization | 132 (84.6%) | ||
| vaccinated but unknown timing relative to hospitalization | 4 (2.6%) | ||
| Required ICU stay for COVID-19 infection | N = 47 (5.7%) | P < 0.001 | |
| history of ≥ 1 vaccine prior to ICU | 6 (12.8%) | ||
| no vaccination prior to ICU | 41 (87.2%) | ||
| vaccinated but unknown timing relative to ICU stay | 0 (0%) | ||
| Died in association with COVID-19 infection | N = 28 (3.4%) | P < 0.001 | |
| history of ≥ 1 vaccine prior to death | 5 (17.9%) | ||
| no vaccination prior to death | 23 (82.1%) | ||
| vaccinated but unknown timing relative to death | 0 (0%) |
Figure 2.
Hospitalization, ICU admission, and death among USIDNET Registry cohort. Categorization was adapted from International Union of Immunological Societies (IUIS) phenotypic classification. Age quartile (years) is based on patient age at time of COVID-19 infection. Three infected patients lacked data on age. COVID-19 risk factors included history of lung disease, immunosuppressive medication use in the 3 months preceding infection, obesity, and renal disease. Additionally, a measure of “other risk factors” was determined, representing a composite of uncommonly observed risk factors in the cohort - neuromuscular disease, tracheostomy, heart disease, sickle cell disease, and diabetes. Any patient with at least one of these uncommonly observed risk factors was counted for this measure. Vaccination was determined as receipt of at least 1 COVID-19 vaccine prior to COVID-19 infection. Sixty-six patients lacked adequate information on timing of vaccination relative to infection and were not included.
Table 5.
Characteristics of deceased patients.
| Age | Diagnosis | Comorbidities and Other Conditions | Vaccination Status | Cause of Death | Δ^ |
|---|---|---|---|---|---|
| 85 | CVID | GLILD, CLL, primary ITP | vaccinated | acute hypoxic respiratory failure due to COVID pneumonia, septic shock | 9 |
| 76 | CVID | obesity, chronic kidney disease, diabetes mellitus, coronary artery disease, GLILD | unvaccinated | ARDS secondary to COVID-19 | 11 |
| 75 | IgG Subclass | diabetes mellitus, AIHA | unvaccinated | COVID pneumonia, ARDS, multiorgan failure | 21 |
| 74 | CVID | asthma, obesity, atrophic gastritis, hypertension | unvaccinated | COVID-19 | 6 |
| 74 | CVID | asthma, dysphagia, granulomatosis | unvaccinated | respiratory failure | 10 |
| 74 | CVID | obesity, pulmonary granuloma | vaccinated | COVID-19 pneumonia | 14 |
| 54 | CVID | ITP, bronchiectasis | unvaccinated | respiratory failure | 21 |
| 50 | CVID | bronchiectasis, diarrhea, cirrhosis, portal hypertension | vaccinated | pneumonia, respiratory failure | 9 |
| 49 | CVID | bronchiectasis | vaccinated | multiorgan failure | 88 |
| 41 | CVID | ITP, bronchiectasis, GLILD, lymphoproliferative disorder, portal HTN, lymphoma with secondary HLH | unvaccinated | COVID-19 respiratory failure | 12 |
| 39 | hypogammaglobulinemia | kidney transplant, T cell leukemia/lymphoma, Hodgkin lymphoma | unvaccinated | multiorgan failure, shock | 35 |
| 28 | Kabuki | interstitial lung disease | unvaccinated | respiratory failure | 26 |
| 28 | unspecified agammaglobulinemia | none | vaccinated | unknown | 31 |
| 28 | XLA | bronchiectasis | unvaccinated | ARDS due to COVID-19, bacterial pneumonia, sepsis | 9 |
| 26 | XLA | none | unvaccinated | respiratory failure | unknown |
| 18 | KMT2A | asthma, CLD, chronic respiratory failure on BiPAP, epilepsy, dysphagia | unvaccinated | respiratory failure | 3 |
| 18 | HLH (transplanted) | encephalitis, hemolytic anemia, TMA | unvaccinated | pneumonia, COVID-19 | 50 |
| 15 | APECED | hypoparathyroidism, Addison Disease, vitiligo, thyroiditis, asplenia, hypocalcemia | unvaccinated | pneumonia, pulmonary failure, nosocomial fungal sepsis | 39 |
| 14 | LRBA Deficiency | inflammatory bowel disease, AIHA, ITP, EBV, CMV colitis, arthritis, obliterative bronchiolitis, asthma | unvaccinated | E. Coli sepsis | unknown |
| 12 | CVID | malnutrition, bronchiectasis, TTP, diarrhea | unvaccinated | respiratory failure | 36 |
| 12 | STK4 | meningitis, cellulitis, ITP, AIHA, lymphadenopathy, seizures | unvaccinated | respiratory failure, cardiac failure | 10 |
| 9 | TPP2 Deficiency | ataxia, chronic idiopathic thrombocytopenic purpura | unvaccinated | respiratory failure, DIC | 10 |
| 7 | HLH (not transplanted) | none | unvaccinated | HLH | 87 |
| 3 | Combined immune deficiency | childhood bullous pemphigoid, candidiasis, failure to thrive | unvaccinated | sepsis | 37 |
| 3 | Unspecified autoinflammatory disorder | psoriasis with arthropathy | unvaccinated | sepsis, ARDS | 17 |
| 3* | IFNAR1 mutation | chronic sinusitis, thrush, mucormycosis | unvaccinated | respiratory failure, left ventricular dysfunction/arrythmias | 51 |
| 1 | NEMO (transplanted) | norovirus enteritis, adenoviral gastroenteritis | unvaccinated | Stenotrophomonas sepsis | 76 |
| < 1 | SCID (not transplanted) | none | unvaccinated | sepsis, COVID-19 pneumonia | 59 |
all vaccinated subjects had vaccine prior to acute infection
ages listed in years
subject developed MIS-C in addition to acute COVID-19.
Δ = diagnosis date - death date, given in days
Hospitalization, ICU admission, and death rates varied by IUIS diagnosis group (FIGURE 2). The highest rates were observed in patients with innate immune defects with hospitalization observed in 44%, ICU admission observed in 22%, and death observed in 11% of these patients. Patients with combined immune deficiencies, immune dysregulation, and auto-inflammatory disorders also had higher rates of hospitalization, ICU admission, and death (FIGURE 2). Lower rates were observed in patients with antibody deficiencies, and the lowest rates were observed in patients with phagocyte deficiencies and complement deficiencies, with no ICU admissions or deaths observed in those two patient groups. Rates of hospitalization, ICU admission, and death were higher in patients with co-morbidities and in patients in the oldest age quartile (FIGURE 2).
Effect of Vaccination on COVID-19 Outcomes
Of 806 patients who received one or more vaccinations against SARS-CoV-2, 216 patients received vaccination prior to COVID-19 infection, 541 were vaccinated after COVID-19 infection, and timing of vaccination was not clear in the remaining patients. Patients who received vaccination after COVID-19 infection were counted in the unvaccinated group for analyses, and subjects with uncertain timing of vaccination relative to infection were excluded.
Rates of hospitalization, ICU admission, and death were all proportionately lower in the patients who received 1 or more vaccinations prior to COVID-19 infection (FIGURE 2). Twenty of 216 (9.3%) IEI patients who received 1 or more vaccinations prior to COVID-19 infection were hospitalized, compared with 132/541 (24.4%) who had not received at least 1 vaccine (p < 0.001). Six of 216 (2.8%) vaccinated patients were admitted to the ICU, compared with 41 of 541 (7.6%) unvaccinated patients (p = 0.013). Five of 216 (2.3%) vaccinated patients died and 23 of 541 (4.3%) unvaccinated patients died (p = 0.202).
In unadjusted logistic regression analysis (Table 6), not having at least one COVID-19 vaccine prior to first COVID-19 infection significantly increased the odds of non-ICU admission by a factor of 3.16 (95% CI (1.92–5.22), p < 0.001) and the odds of ICU admission by a factor of 2.87 (95% CI (1.20–6.86), p = 0.018). Although the odds of death were also increased in the unvaccinated group, this difference was not significantly significant.
Table 6.
Logistic regression analysis for COVID-19-related hospitalization, ICU admission, and death.
| Unadjusted Analysis | |||
| Outcome: Hospitalization (n = 757) * | |||
| Variable | Odds Ratio | 95% CI | P value |
| no COVID vaccine | 3.16 | 1.92–5.22 | < 0.001 |
| Outcome: ICU Admission (n = 757) * | |||
| Variable | Odds Ratio | 95% CI | P value |
| no COVID vaccine | 2.87 | 1.20–6.86 | 0.018 |
| Outcome: Death (n = 757) * | |||
| Variable | Odds Ratio | 95% CI | P value |
| no COVID vaccine | 1.87 | 0.70–4.99 | 0.209 |
| Adjusted Analysis | |||
| Outcome: Hospitalization (n = 748) ^ | |||
| Variable | Point Estimate | 95% CI | P value |
| no COVID vaccine | 3.84 | 2.28–6.49 | < 0.001 |
| composite risk factors | 1.08 | 0.34–3.46 | 0.901 |
| obesity | 1.26 | 0.72–2.21 | 0.421 |
| renal | 1.40 | 0.61–3.19 | 0.426 |
| immunosuppressive meds | 1.37 | 0.85–2.20 | 0.196 |
| lung | 1.55 | 1.06–2.25 | 0.023 |
| age | 1.01 | 1.00–1.02 | 0.005 |
| Outcome: ICU Admission (n = 748) ^ | |||
| Variable | Point Estimate | 95% CI | P value |
| no COVID vaccine | 3.61 | 1.48–8.79 | 0.005 |
| composite risk factors | 0.94 | 0.12–7.45 | 0.956 |
| obesity | 0.30 | 0.07–1.28 | 0.103 |
| renal | 0.76 | 0.17–3.41 | 0.719 |
| immunosuppressive meds | 2.26 | 1.13–4.50 | 0.021 |
| lung | 1.43 | 0.77–2.63 | 0.256 |
| age | 1.02 | 1.00–1.03 | 0.011 |
| Outcome: Death (n = 748) ^ | |||
| Variable | Point Estimate | 95% CI | P value |
| no COVID vaccine | 2.30 | 0.85–6.28 | 0.103 |
| composite risk factors | 1.63 | 0.20–13.09 | 0.645 |
| obesity | 0.87 | 0.25–3.00 | 0.827 |
| renal | 0.58 | 0.07–4.53 | 0.602 |
| immunosuppressive meds | 2.69 | 1.17–6.19 | 0.020 |
| lung | 1.59 | 0.73–3.45 | 0.239 |
| age | 1.02 | 1.00–1.04 | 0.048 |
823 patients had a COVID-19 infection at least once
Of these, 66 patients were vaccinated but lacked information on timing of vaccine relative to COVID-19 illness and were thus excluded from unadjusted analysis.
823 patients had a COVID-19 infection at least once.
Of these, 66 patients were known to be vaccinated but lacked information on timing of vaccination relative to COVID-19 illness, and an additional 9 were missing data on age. These were excluded from adjusted analysis.
Similarly, we performed regression analysis for the same outcomes, but adjusting for the potential confounders age, obesity, renal disease, immunosuppressive medication use, lung disease, and other composite risk factors (Table 6). Not having at least one COVID-19 vaccine prior to first COVID-19 infection significantly increased the odds of non-ICU admission (OR 3.84, 95% CI (2.28–6.49), p < 0.001) and ICU admission (OR 3.61, 95% CI (1.48–8.79), p = 0.005). While odds of death were increased in the nonvaccinated group (OR 2.30, 95% CI (0.85–6.28)), this difference was not statistically significant (p = 0.103. There was also a small but significant effect on the odds of hospitalization, ICU admission, and death for each increase in year of age (Table 6). Lung disease significantly impacted risk of hospitalization, and immunosuppressive medication use significantly impacted risk of ICU admission and death.
Discussion
This is the largest registry report of COVID-19 vaccination and/or infection in IEI patients (n = 1,245) to date. The disease burden in this multinational cohort of patients was diverse, with representation of even very rare diseases in each IUIS category.
Our study demonstrates that COVID-19 infections were most commonly mild in this phenotypically diverse patient population. Over 95% of patients can be expected to survive COVID-19 infection. However, a significant proportion of COVID-19 infected IEI patients required hospitalization (19%) and ICU care (5.7%), and a minority did succumb (3.4%). The observed COVID-19 death rate in this large IEI registry cohort, which is largely US-based, is higher than the US COVID-19 death rate in general (1.1%) and approaches that seen in medically-underserved Ecuador (3.6%) (23). Similar to previous studies, we observed that patients with innate immune defects, combined immunodeficiencies, disorders of immune dysregulation, and autoinflammatory disorders appear to have higher rates of severe complications of COVID-19 compared to patients with antibody deficiencies, phagocyte disorders, and complement deficiencies (4, 5, 24). However, previous reports have included higher complication and death estimates in the IEI population than observed in our registry cohort. A recent systematic review on COVID-19 in patients with primary immunodeficiency found a case fatality rate of 9% and hospitalization rate of 49% (24). These differences may be due to the different populations of the patients, with US patients representing a minority of patients in the review versus 63.5% of our USIDNET registry cohort. Abolhassani and colleagues performed a review of the COVID-19/IEI literature and found severe COVID-19 presentations in 21.5% of IEI patients, and COVID-19 related mortality in 8.3% (7). Of note, however, many of these cases of SARS-CoV-2 infection occurred prior to the widespread availability of vaccinations, and the Abolhassani cohort included more innate immune deficiencies which have been linked to more severe outcomes. A study based in the UK by Shields and colleagues found even higher rates of hospitalization (53.3%) and case-fatality (39.2%), although this cohort included patients with secondary immune deficiencies in addition to IEI patients (25). In contrast, an Italian IEI/COVID-19 study by Milito and colleagues reported a comparable infection mortality rate (3.8%) to that observed in our cohort (26) as did the Cousins study (3%) although the latter found a much larger difference in odds of hospitalization (18). As before, the heterogeneity in these estimates is likely secondary to various confounding effects – temporal factors such as predominant SARS-CoV-2 variant at different times and increasing access to vaccination over time, cohort-level factors including diagnosis breakdown, and varying inclusion-exclusion criteria.
Vaccination in the IEI population was noted in our study to be quite effective in preventing COVID-19 general hospitalization and ICU admission, with > 3.5 times increased odds ratios for these outcomes in the unvaccinated group compared to those with at least one vaccine dose in adjusted regression analyses. Notably, the odds of death were not significantly decreased in the COVID-19 vaccinated group – a fact that is likely attributable to the generally low numbers of deaths in the cohort (28 patients), and even lower number of vaccinated deaths (5 patients), affecting statistical power.
Adverse effects of vaccination were generally mild and occurred in < 3.5% of vaccinated patients, further supporting the use of COVID-19 vaccination in patients with IEI. For the few vaccinated patients who required escalation of care after vaccination, it is not clear whether or how the vaccination event itself may have contributed to this. Importantly, there were no cases of anaphylaxis or vaccine-induced myocarditis in the cohort, though this does not rule out the occurrence. Myocarditis has been reported following a third mRNA vaccination in a 17 year old male with CVID, for instance, and anaphylaxis would certainly be expected to occur in a minority of patients (27).
Limitations of the study include several inherent in registry-based observational research. These include recall bias on the part of clinicians filling out the survey, as well as ascertainment bias in terms of cases included. Additionally, respondents entered surveys at one point in time and were unable to update their entries later. Thus, information on repeat COVID-19 infections/outcomes and repeat COVID-19 vaccinations/outcomes was not captured. To facilitate analysis, subjects with more than one COVID-19 vaccine were pooled with those receiving only one, although it is reasonable to suppose that the degree of protection was different between these groups. Finally, the study design precluded evaluating for differences in rates of COVID-19 infection in vaccinated and unvaccinated patients. However, the apparent impact of vaccination on improving COVID-19 outcomes and the generally observed safety are important observations that may encourage hesitant IEI patients (up to 42%) to receive vaccination (28).
In summary, our study of a largely U.S.-based registry cohort demonstrates that COVID-19 infections are mild in most patients with IEI but can be severe, and the percentages of serious COVID-19 outcomes (hospitalization, ICU care, or death) in this medically vulnerable group remain substantial. Vaccination appears safe and effective in decreasing serious outcomes among patients with diverse IEI.
Acknowledgements
The authors wish to thank Claudia Terrazas Saavedra for her contributions to the project.
Funding
This study was funded by a grant from the National Institutes of Health National Institute of Allergy and Infectious Diseases, R24AI171055.
Abbreviations
- SCID
severe combined immune deficiency
- CID
combined immune deficiency
- A-T
ataxia-telangiectasia
- WAS
Wiskott-Aldrich syndrome
- CHARGE
coloboma/heart defects/atresia choanae/growth retardation/genital abnormalities/ear abnormalities
- NEMO
nuclear factor-kappa B essential modulator deficiency
- CVID
common variable immune deficiency
- hypogamma
hypogammaglobulinemia
- agamma
agammaglobulinemia
- Comp. Def.
complement deficiency
- SAD
specific antibody deficiency
- Subclass Def.
IgG subclass deficiency
- IgA Def.
IgA Deficiency
- HLH/EBV Susc.
hemophagocytic lymphohistiocytosis and EBV susceptibility
- ALPS
autoimmune lymphoproliferative syndrome
- IPEX
immune dysregulation/polyendocrinopathy/enteropathy/X-linked syndrome
- VEO-IBD
very early onset inflammatory bowel disease
- CGD
chronic granulomatous disease
- MSMD
Mendelian susceptibility to mycobacterial disease
- Cong. Neut.
congenital neutropenia
- Marrow Fail.
bone marrow failure
- Viral Predisp.
predisposition to severe viral infection
Footnotes
Conflicts of Interests/Competing Interests
Deepti Deshpande is employed by Regeneron Pharmaceuticals, spouse employed by Arch Oncology. Elizabeth Ristagno owns stock in Moderna and Pfizer. Kathleen Sullivan is a consultant for the Immune Deficiency Foundation. Rebecca Marsh is an employee of Pharming Healthcare, Inc.
Ethics Approval
The study was approved as exempt research by the Cincinnati Children’s Hospital institutional review board (IRB ID: 2021–0406).
Consent to Participate
Not applicable
Consent to Publish
Not applicable
Availability of Data and Material
Researchers interested in access to the data may contact John McDonnell at mcdonnj@ccf.org.
References
- 1.V’Kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19(3):155–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–42. [DOI] [PubMed] [Google Scholar]
- 3.Kompaniyets L, Pennington AF, Goodman AB, Rosenblum HG, Belay B, Ko JY et al. Underlying Medical Conditions and Severe Illness Among 540,667 Adults Hospitalized With COVID-19, March 2020–March 2021. Prev Chronic Dis. 2021;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyts I, Bucciol G, Quinti I, Neven B, Fischer A, Seoane E, et al. Coronavirus disease 2019 in patients with inborn errors of immunity: An international study. J Allergy Clin Immunol. 2021;147(2):520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delmonte OM, Castagnoli R, Notarangelo LD. COVID-19 and Inborn Errors of Immunity. Physiol (Bethesda). 2022;37(6):0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370(6515). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abolhassani H, Delavari S, Landegren N, Shokri S, Bastard P, Du L et al. Genetic and immunologic evaluation of children with inborn errors of immunity and severe or critical COVID-19. J Allergy Clin Immunol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferre EMN, Schmitt MM, Ochoa S, Rosen LB, Shaw ER, Burbelo PD, et al. SARS-CoV-2 Spike Protein-Directed Monoclonal Antibodies May Ameliorate COVID-19 Complications in APECED Patients. Front Immunol. 2021;12:720205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tangye SG. consortium CHGE. Impact of SARS-CoV-2 infection and COVID-19 on patients with inborn errors of immunity. J Allergy Clin Immunol. 2023;151(4):818 – 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergman P, Blennow O, Hansson L, Mielke S, Nowak P, Chen P, et al. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. EBioMedicine. 2021;74:103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pulvirenti F, Fernandez Salinas A, Milito C, Terreri S, Piano Mortari E, Quintarelli C et al. B Cell Response Induced by SARS-CoV-2 Infection Is Boosted by the BNT162b2 Vaccine in Primary Antibody Deficiencies. Cells. 2021;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gernez Y, Murugesan K, Cortales CR, Banaei N, Hoyte L, Pinsky BA, et al. Immunogenicity of a third COVID-19 messenger RNA vaccine dose in primary immunodeficiency disorder patients with functional B-cell defects. J Allergy Clin Immunol Pract. 2022;10(5):1385–8. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delmonte OM, Bergerson JRE, Burbelo PD, Durkee-Shock JR, Dobbs K, Bosticardo M, et al. Antibody responses to the SARS-CoV-2 vaccine in individuals with various inborn errors of immunity. J Allergy Clin Immunol. 2021;148(5):1192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dan J, da Silva Antunes R, Grifoni A, Weiskopf D, Crotty S, Sette A. Observations and Perspectives on Adaptive Immunity to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2022;75(Supplement1):24–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pham MN, Murugesan K, Banaei N, Pinsky BA, Tang M, Hoyte E, et al. Immunogenicity and tolerability of COVID-19 messenger RNA vaccines in primary immunodeficiency patients with functional B-cell defects. J Allergy Clin Immunol. 2022;149(3):907–11e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Fusco M, Moran MM, Cane A, Curcio D, Khan F, Malhotra D, et al. Evaluation of COVID-19 vaccine breakthrough infections among immunocompromised patients fully vaccinated with BNT162b2. J Med Econ. 2021;24(1):1248–60. [DOI] [PubMed] [Google Scholar]
- 18.Cousins K, DeFelice N, Jeong S, Feng J, Lee ASE, Rotella K, et al. SARS-COV-2 infections in inborn errors of immunity: A single center study. Front Immunol. 2022;13:1035571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimabukuro TT, Cole M, Su JR. Reports of Anaphylaxis After Receipt of mRNA COVID-19 Vaccines in the US-December 14, 2020-January 18, 2021. JAMA. 2021;325(11):1101–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR, et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA. 2022;327(4):331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cetin Gedik K, Lamot L, Romano M, Demirkaya E, Piskin D, Torreggiani S, et al. The 2021 European Alliance of Associations for Rheumatology/American College of Rheumatology points to consider for diagnosis and management of autoinflammatory type I interferonopathies: CANDLE/PRAAS, SAVI and AGS. Ann Rheum Dis. 2022;81(5):601–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bousfiha A, Moundir A, Tangye SG, Picard C, Jeddane L, Al-Herz W, et al. The 2022 Update of IUIS Phenotypical Classification for Human Inborn Errors of Immunity. J Clin Immunol. 2022;42(7):1508–20. [DOI] [PubMed] [Google Scholar]
- 23.Johns Hopkins Coronavirus. Resource Center - Data Visualizations.
- 24.Drzymalla E, Green RF, Knuth M, Khoury MJ, Dotson WD, Gundlapalli A. COVID-19-related health outcomes in people with primary immunodeficiency: A systematic review. Clin Immunol. 2022:109097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shields AM, Burns SO, Savic S, Richter AG, Consortium UPC-. COVID-19 in patients with primary and secondary immunodeficiency: The United Kingdom experience. J Allergy Clin Immunol. 2021;147(3):870–5e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milito C, Lougaris V, Giardino G, Punziano A, Vultaggio A, Carrabba M, et al. Clinical outcome, incidence, and SARS-CoV-2 infection-fatality rates in Italian patients with inborn errors of immunity. J Allergy Clin Immunol Pract. 2021;9(7):2904–6. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozdemir O, Seker E, Dikici U, Gunes M. Myocarditis development after COVID-19 vaccination in an immunodeficient case. Immunol Lett. 2023;260:22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pergent M, Haerynck F, Hoste L, Gardulf A. COVID-19 vaccination in patients with primary immunodeficiencies: an international survey on patient vaccine hesitancy and self-reported adverse events. Front Immunol. 2023;14:1166198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Researchers interested in access to the data may contact John McDonnell at mcdonnj@ccf.org.