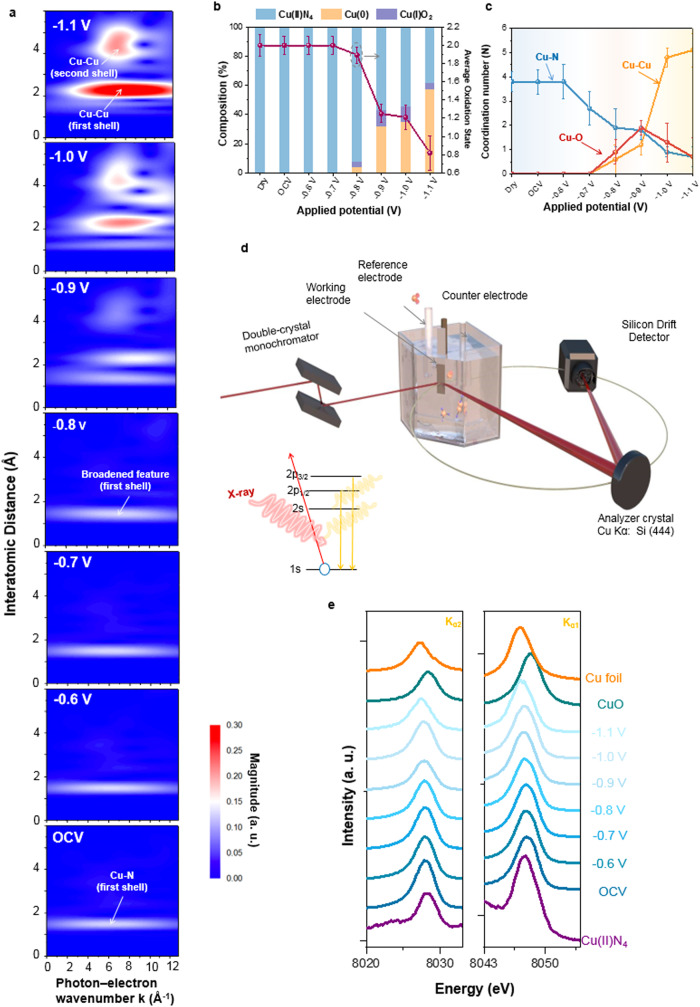
Fig. 2. In situ X-ray spectroscopy analysis of N–Cu SAC during CO2RR.
a Wavelet-transform diagram of in situ Cu K-edge EXAFS spectra of N–Cu SAC at various applied potentials in CO2-saturated 0.1 M KHCO3 solution during CO2 reduction. b Extracted oxidation state from in situ Cu K-edge XANES spectra of N–Cu SAC through linear combination of Cu foil, Cu2O, and Cu(bipy)4Cl as spectroscopic references in CO2-saturated 0.1 M KHCO3 solution. c Quantitative analysis of coordination environment extracted from EXAFS fitting at various potentials in CO2-saturated 0.1 M KHCO3 solution. Error bars represent the standard deviation of three independent measurements. d Schematic representation of in situ X-ray emission spectroscopy (XES) apparatus applied to a liquid electrochemical cell and energy diagram for the detection of Kα1 and Kα2 emission in transition-metal ion. e In situ XES spectra of Kα1 and Kα2 emission for N–Cu SAC and references collected at various potentials during CO2 reduction in CO2-saturated 0.1 M KHCO3 solution. All measurements were performed in 0.1 M KHCO3 by a typical three-electrode setup using glassy carbon as the working electrode, Hg/HgCl2 electrode and platinum plate acted as reference and counter electrodes, respectively. The applied potential was calibrated to a reversible hydrogen electrode (RHE).