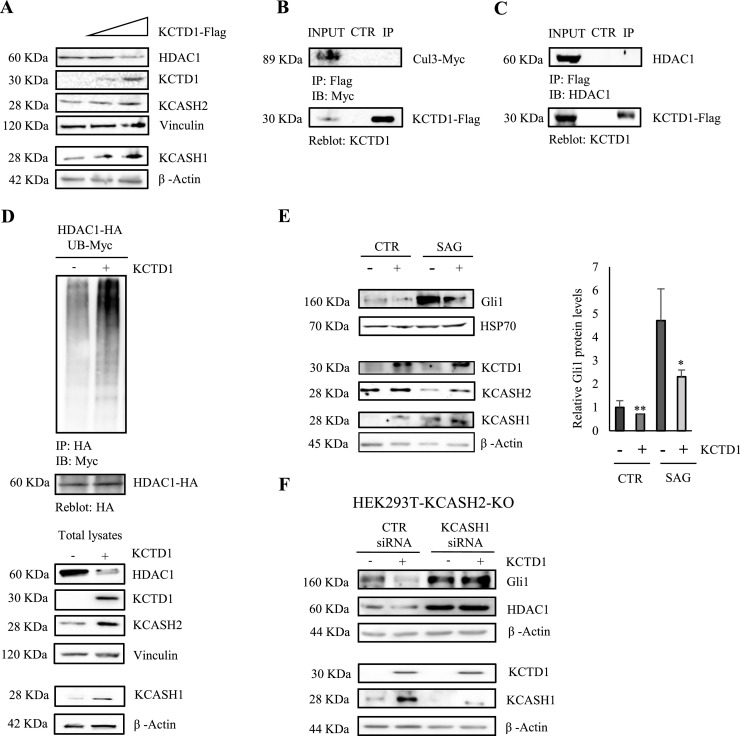
Fig. 5.
KCTD1 reduces Gli1 transcriptional activity and HDAC1 protein levels but does not interact with Cul3 and HDAC1. (A) KCTD1 expression reduces HDAC1 protein levels. HEK293T cells were transfected with different amounts of KCTD1; after 24 h, cells were lysed and endogenous HDAC1, KCASH1 and KCASH2 protein levels were analysed by WB. Vinculin and β-Actin were used as loading controls. KCTD1 is not able to interact with Cul3 (B) and HDAC1 (C). Co-IP assays were performed from HEK293T cells transfected with expression vector Flag-tagged KCTD1, Myc-tagged Cul3 (B); from HEK293T cells transfected with expression vector Flag-tagged KCTD1 (C). Total lysates were immunoprecipitated (IP) with anti-Flag agarose beads and IP samples and a fraction of the total lysate were separated on SDS-PAGE gels. Blots were immunoblotted (IB) with anti-HDAC1 antibody, anti-Cul3 antibody, and reblotted with anti-KCTD1 antibody. (D) On the top, the KCTD1 presence promotes HDAC1 ubiquitination. Lysates from HEK293T cells, transfected with the indicated plasmids, were immunoprecipitated with anti-HA agarose beads and immunoblotted with anti-Myc to detect conjugated Myc-Ub. The blot was re-probed with an anti-HA antibody to monitor immunoprecipitation efficiency. On the bottom, HDAC1, KCTD1, KCASH2 and KCASH1 total protein levels. Vinculin and β-Actin protein were used as loading controls. (E) KCTD1 is not able to decrease Hedgehog activity in absence of KCASH1 and KCASH2. HEK293T KCASH2-KO cells were transfected with scrambled siRNA (siCTR) or with KCASH1 siRNA (siKCASH1) and 48 h later protein lysates were analysed by WB, using HDAC1, KCTD1, KCASH1 and Gli1 antibodies. β-Actin protein was used as a normalizer. (F) KCTD1 reduces Gli1 activity in cells treated with Smo agonist SAG. HEK293T were transfected with empty vector or KCTD1 expressing vector, pre-starved in serum-free medium for 24 h and then treated with 200nM SAG for 48 h. Protein lysates were analysed by WB, using KCTD1, Gli1, KCASH2 and KCASH1 antibodies. β-Actin protein was used as loading control.