Figure 1. Mitochondrial dysfunction and oxidative stress in SLE.
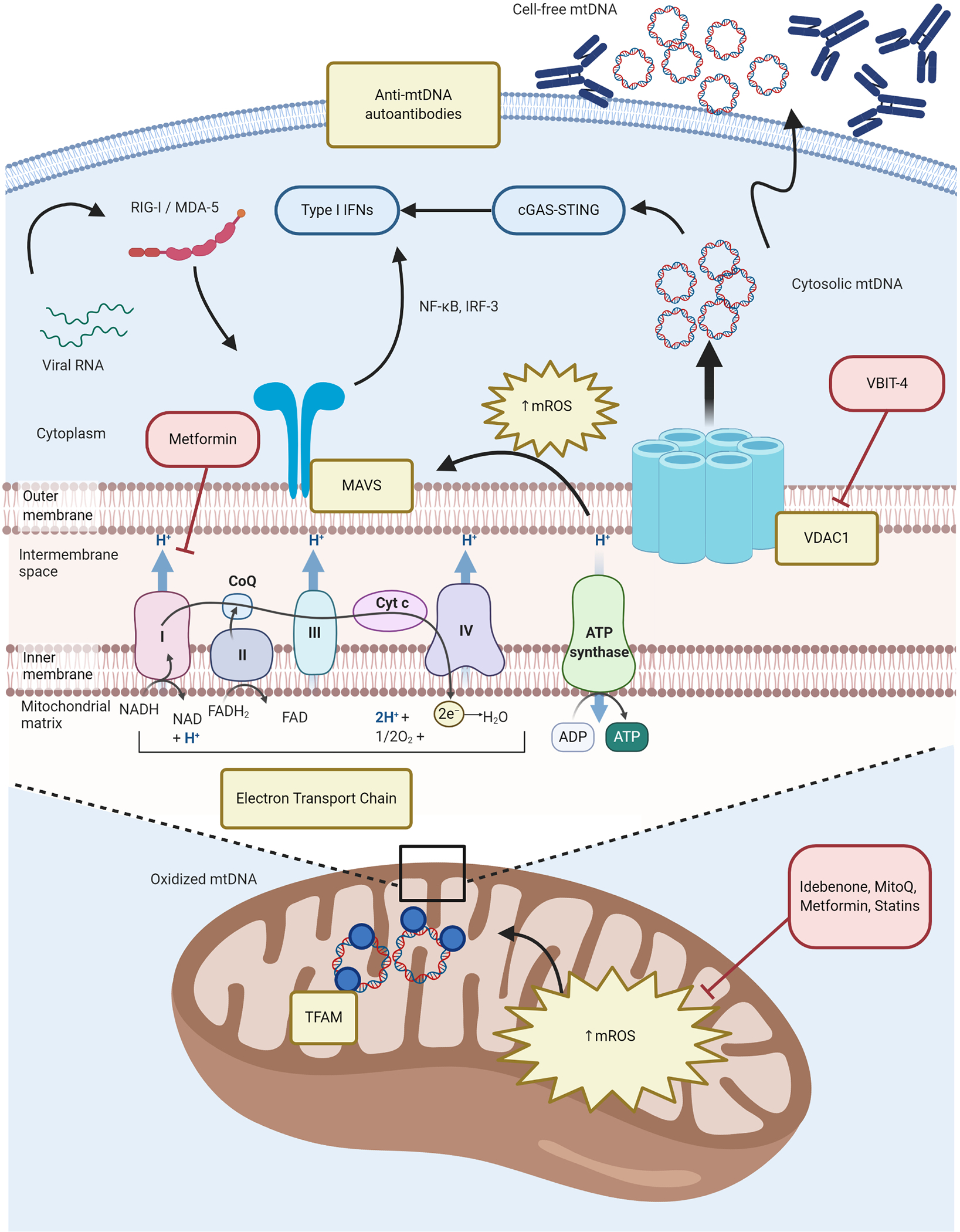
Chronically increased OXPHOS in immune cells promotes enhanced generation of mROS and enhanced type I IFN responses. Excessive mROS synthesis promotes MAVS oligomerization in the MOM that is essential for antiviral immune responses but dysregulated in SLE. Enhanced oxidative stress along with a putative defective disassociation of mtDNA from TFAM in the mitochondrial matrix, facilitates the oxidation of mtDNA. Increased VDAC oligomers in the MOM interact with oxidized mtDNA fragments and facilitating their release into the cytoplasm, where they activate the cGAS-STING pathway and upregulate ISG expression. Additionally, autoantibodies to free-cell mtDNA may contribute to lupus immune dysregulation. Perturbations in immunometabolism pathways described in SLE are depicted in yellow boxes; novel therapeutic strategies targeting some of these specific defects are depicted in red boxes. cGAS-STING = cyclic guanosine monophosphate-–adenosine monophosphate synthase (cGAS)-Stimulator of Interferon Genes (STING); CoQ = coenzyme Q10; Cyt c = cytochrome c; IFNs = interferons; IRF-3 = Interferon regulatory factor 3; MAVS = mitochondrial antiviral stimulator; MDA-5 = melanoma differentiation-associated protein 5; mROS = mitochondrial reactive oxygen species (ROS); mtDNA = mitochondrial DNA; NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells; OXPHOS = oxidative phosphorylation; RIG-I = retinoic acid-inducible gene I; SLE = systemic lupus erythematosus; VDAC1 = voltage-dependent anion channel 1.
