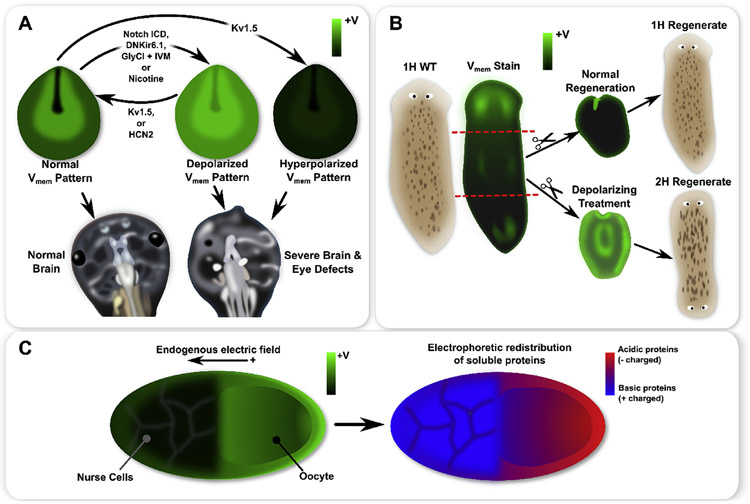
Fig. 1.
A schematic of functional patterns observed in frog and insect development, as well as planaria regeneration. Xenopus laevis neurula exhibit a characteristic pattern with strong hyperpolarization in cells of the closing neural tube and depolarization in the surrounding neural folds (A). Genetic mis-expression of specific ion channels or pharmacological treatments can flatten the endogenous pattern by creating a more uniform depolarization or hyperpolarization, where both induced severe brain and eye defects (A). Moreover, for depolarization treatments (e.g. Notch ICD or nicotine), expression of Kv1.5 or HCN2 restored the normal pattern and produced normal brain and eye development (A). In whole planaria flatworms (B), an anterior-posterior gradient of exists with significantly stronger depolarization at the anterior (B). During normal regeneration, a strong anterior depolarization appears, which is believed to be instructive for anterior-posterior axis polarity (B). Depolarizing a fragment during regeneration induces 2H outcomes in regenerates (B). In insect ovarian follicles, an endogenous gradient features an oocyte which is more depolarized than the nurse cells (C). Patterns of soluble acidic (negatively charged) and basic (positively charged) proteins may be correlated with the pattern in the ovarian follicle, with more acidic proteins found in the oocyte and more basic proteins found in the nurse cells, thereby supporting the hypothesis that small charged molecules travel by electrodiffusion across intercellular connections such as gap junctions (C).