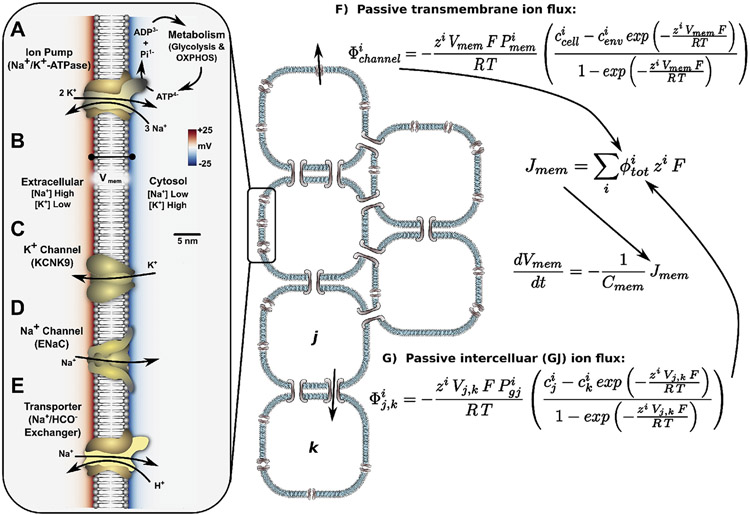
Fig. 2.
A summary of basic concepts underlying generation, modulation, and dynamics in a gap junction-coupled somatic cell network. Ion pumps such as the ubiquitous Na+,K+-ATPase (A) use chemical energy released in ATP hydrolysis to maintain concentration gradients of Na+ (low inside the cell) and K+ (high inside the cell) across the plasma membrane, and generate an electronegative (B). Ion channels (C, D) provide ion-specific pores in the membrane to allow ions to passively move down their electrochemical gradients, altering in the process by changing the net charge distribution across the membrane. The open/closed state of ion channels can be modulated by (voltage-sensitive channels) or chemicals (ligand-gated channels), which in combination with the altering effects of ion channels, introduces the possibility for positive and negative feedbacks to the bioelectrochemical system. Transporters, such as the Na+/HCO3− exchanger (E), utilize the potential energy of electrochemical gradients generated by ion pumps to perform a host of functions including maintenance of balanced pH, levels of glucose and metabolites; regulation of neurotransmitter signaling (e.g. monoamine reuptake transporters); and a host of other functions. Passive transmembrane ion flux for any channel can be described in terms of chemical and electrical gradients across the membrane (F), with a similar description for ion flux between two gap junction-coupled cells (G).