Figure 4.
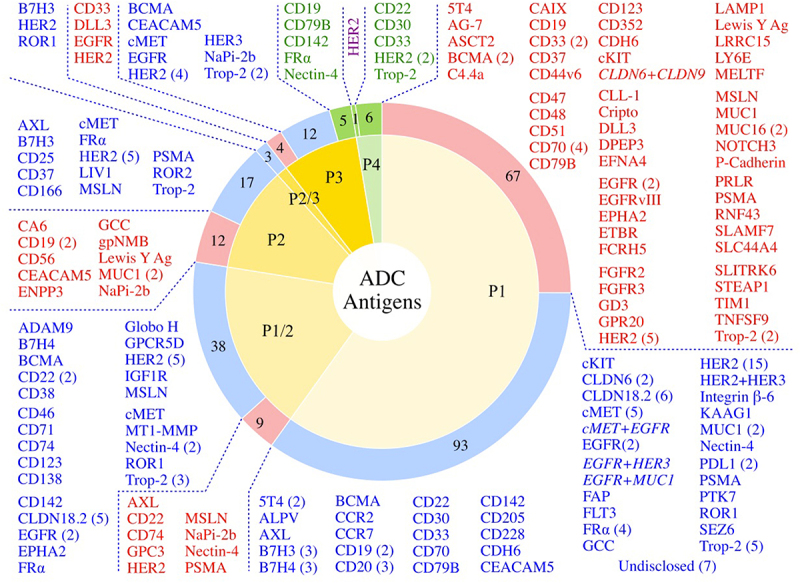
Antigen Targets of the Clinically Tested ADCs. Of the 267 clinically tested ADCs, 260 have known antigens (7 are undisclosed). Numbers of ADCs targeting a given tumor antigen in various stages of clinical testing (Phase 1-Phase 4, P1-P4) are shown in the categories of FDA Approved ADCs (green sectors, green text), Active ADCs (blue sectors, blue text), and Discontinued ADCs (red sectors, red text). Dual antigen targeting ADCs are shown in italics. The Phase 4 HER2 candidate shown in purple text is disitamab vedotin, that has been approved in China and is not yet approved by the FDA.
