Abstract
One of the most promising tools for the control of fungal plant diseases is spray-induced gene silencing (SIGS). In SIGS, small interfering RNA (siRNA) or double-stranded RNA (dsRNA) targeting essential or virulence-related pathogen genes are exogenously applied to plants and postharvest products to trigger RNA interference (RNAi) of the targeted genes, inhibiting fungal growth and disease. However, SIGS is limited by the unstable nature of RNA under environmental conditions. The use of layered double hydroxide or clay particles as carriers to deliver biologically active dsRNA, a formulation termed BioClay™, can enhance RNA durability on plants, prolonging its activity against pathogens. Here, we demonstrate that dsRNA delivered as BioClay can prolong protection against Botrytis cinerea, a major plant fungal pathogen, on tomato leaves and fruit and on mature chickpea plants. BioClay increased the protection window from 1 to 3 weeks on tomato leaves and from 5 to 10 days on tomato fruits, when compared with naked dsRNA. In flowering chickpea plants, BioClay provided prolonged protection for up to 4 weeks, covering the critical period of poding, whereas naked dsRNA provided limited protection. This research represents a major step forward for the adoption of SIGS as an eco-friendly alternative to traditional fungicides.
Keywords: BioClay, double stranded RNA, layered double hydroxide, RNA interference, spray-induced gene silencing
INTRODUCTION
Plant fungal pathogens are an ever-present threat to global food security and can cause devastating yield losses both pre- and post-harvest (Savary et al., 2019). Current fungal disease management strategies are largely reliant on the use of chemical fungicides, host resistance and cultural practices such as crop rotation. However, fungicide usage can lead to the target species developing resistance and synthetic fungicides can be harmful to humans, beneficial organisms and the broader environment (Van de Wouw et al., 2021). Alarmingly, fungicide-resistant fungal strains have been reported for every major class of antifungals used in agriculture (Fisher et al., 2018). In addition, consumers are increasingly demanding lower traces of fungicides and other chemicals on food, triggering policy changes around the world. In June 2022, the European Commission published a proposal for new regulation on the sustainable use of pesticides, which will set legally binding targets to reduce the use and risk of chemical pesticides by 50% by 2030 (European Commission, 2022), as planned under the European Green Deal (European Commission, 2019). However, European growers are concerned about the limited effective and affordable alternatives to date, and regulatory changes in the European Union could end up impacting agricultural exports around the world (Matthews, 2022).
Therefore, to safeguard global food security, ecosystem health and to meet consumer demand, novel, sustainable eco-friendly disease management strategies need to be developed and adopted.
RNA interference (RNAi)-mediated crop protection strategies are a sustainable alternative to chemical pesticides and fungicides (Cai et al., 2018a; Niu et al., 2021). Two approaches which have been successfully utilized against fungal pathogens are host-induced gene silencing (HIGS), where host plants are genetically modified to produce double stranded RNA (dsRNA) targeting selected pathogen/pest genes (Koch and Kogel, 2014), and spray-induced gene silencing (SIGS), where the dsRNA is exogenously applied to pre- or post-harvest plant material (Koch et al., 2016; Wang et al., 2016; McLoughlin et al., 2018; Qiao et al., 2021). Both methods trigger RNAi against the targeted pest/pathogen genes albeit via slightly different mechanisms. In HIGS, plant-produced pathogen targeting siRNAs are thought to be taken up by fungal pathogens during infection through a mechanism known as “Cross-Kingdom RNAi,” a natural process where small RNAs are transferred between plants and pathogens (Wang et al., 2016; Huang et al., 2019; Cai et al., 2021) mainly by extracellular vesicles (Cai et al., 2018b; He et al., 2021; Huang et al., 2021). In SIGS, invading fungal pathogens can take up the dsRNA directly, e.g., from the surfaces of leaves or fruit to silence homologous genes (Wang et al., 2016; Qiao et al., 2021). Both HIGS and SIGS have been tested under field conditions (De Schutter et al., 2022). The SIGS technology developed by Greenlight Biosciences (Ledprona) was successfully tested in field trials, where it provided protection against the Colorado Potato Beetle (Rodrigues et al., 2021).
For SIGS approaches to be commercially feasible, it is imperative that dsRNA stability and durability in the environment is enhanced. This can be facilitated by using carriers such as layered double hydroxide (LDH) or clay particles, which have been extensively used as carriers for several types of biomolecules for drug delivery and in other medical applications (Zhang et al., 2021; Sun et al., 2022). LDH is a unique layered structure which has ion exchange abilities; good biocompatibility; pH sensitivity; biodegradability and high intracellular delivery efficacy (Li et al., 2021). In SIGS applications, spraying plants with dsRNA bound to LDH in a formulation known as BioClay™, has already been shown to afford protection against viruses (Mitter et al., 2017) and insects (Jain et al., 2022). BioClay extended the window of protection against viruses for up to 20 d and improved protection against whitefly eggs and nymphs, which was likely due to the stability and sustained release of the dsRNA when applied as BioClay (Mitter et al., 2017; Jain et al., 2022). LDH conjugated with a construct simultaneously targeting an ergosterol biosynthesis pathway gene, translation elongation factor 2 and chitin synthases 1 genes provided protection against Fusarium oxysporum on tomatoes (Mosa and Youssef, 2021) suggesting fungal control may also be possible with BioClay.
Here, we compared the longevity of BioClay and naked dsRNA-induced protection against Botrytis cinerea, a necrotrophic fungal pathogen that can infect leaves, fruits and flowers of more than 1,000 plant species (Weiberg et al., 2013; Elad et al., 2016; Petrasch et al., 2019) resulting in annual global losses of up to $100 billion (Hua et al., 2018). We demonstrate that BioClay prolongs RNAi-mediated protection against B. cinerea on tomato leaves and fruit, as well as in mature chickpea plants. BioClay-mediated protection from B. cinerea in both pre- and post-harvest plant materials opens up the real-world application of BioClay in the management of fungal diseases of both broad acre and horticultural crops.
RESULTS
Botrytis cinerea spores can efficiently take up dsRNA released from LDH
For SIGS approaches to be effective, RNA molecules need to enter fungal cells. To determine whether B. cinerea spores can take up dsRNA associated with LDH, germinated spores were incubated with BioClay containing fluorescently labeled dsRNA on PDA medium and then examined by confocal microscopy for fluorescence. While dsRNA was rapidly taken up within 3 h, the spores were unable to take up the labeled BioClay-associated dsRNA by 16 h (Figure 1). However, using a rapid dsRNA release protocol to dissociate the LDH and dsRNA in BioClay, the spores were able to take up the labeled RNA within 3 h, and at 16 h, similar levels of fluorescence were observed as with the naked dsRNA (Figure 1).
Figure 1. Fungal cells can take up dsRNA from BioClay™ after dsRNA release.
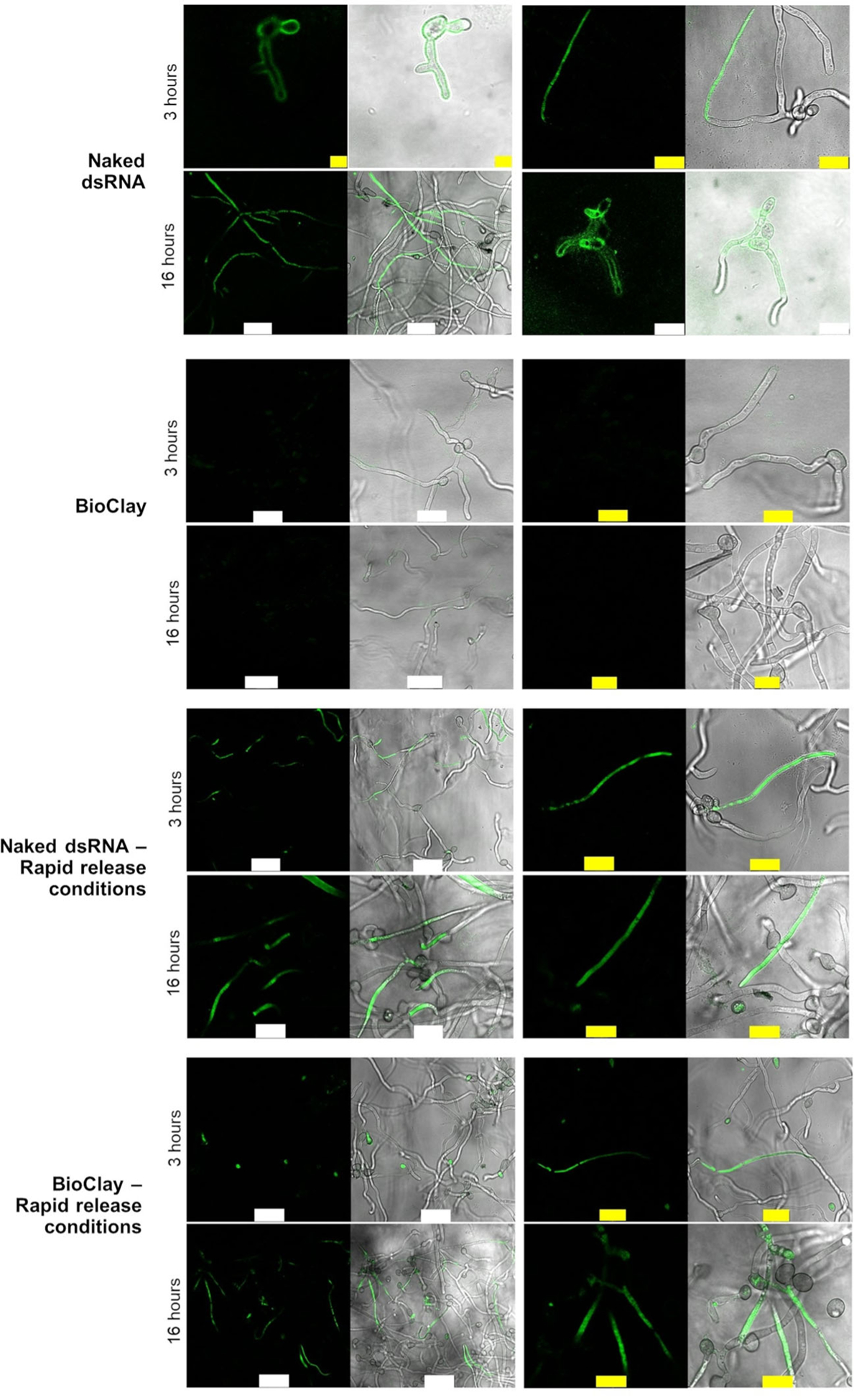
The images show the confocal laser scanning microscopy (CLSM) analysis of germinated spores 3 and 16 h after being treated with fluorescein-labeled dsRNA (YFP or BcDCL1/2). The top group of rows show that Botrytis cinerea can take up the labeled dsRNA on PDA medium at pH ~5.8 after 3 and 16 h. The second group of rows show that dsRNA cannot be taken up when the dsRNA is in the BioClay complex after 3 h. The third group of rows show the rapid release protocol does not affect dsRNA uptake when the dsRNA is in a naked form. The fourth group of rows show that dsRNA can be taken up under conditions that release the dsRNA from the BioClay complex. Micrococcal nuclease (MNase) was added 30 min before capturing the images to remove the external dsRNA, which was not taken up by fungal cells. However, because BioClay provides protection against nucleases, traces of fluorescent signals are observed at the periphery of the fungal cells. White bar = 25 μm, yellow bar = 10 μm.
BioClay provides extended protection against B. cinerea on tomato leaves
To determine whether BioClay can extend the protection window against B. cinerea, we used a detached tomato leaf assay and two dsRNAs that had previously been shown to provide protection against B. cinerea in post-harvest fruit assays: BcDCL1/2 and BcVDS (Wang et al., 2016; Qiao et al., 2021). BcDCL1/2 targets both B. cinerea dicer-like (DCL) genes, while BcVDS targets three genes involved in fungal vesicle trafficking, originally identified as the targets of sRNAs shown to move from Arabidopsis to Botrytis (Cai et al., 2018b). Leaves on intact plants were sprayed with naked dsRNA or BioClay and subsequently detached for inoculation 1–21 d post treatment (dpt). Water, LDH and non-specific dsRNA (cucumber mosaic virus 2b (CMV2b)) controls were also included.
In comparison to the control treatments, both naked dsRNA treatments (BcDCL1/2 and BcVDS) were able to effectively reduce B. cinerea lesion sizes when applied either 1 or 7 d prior to inoculation (Figure 2A, B). However, these treatments did not significantly decrease lesion sizes when applied 14 or 21 d prior to inoculation. Treatments with BcDCL1/2 and BcVDS dsRNA 1 d prior to inoculation resulted in a 62% and 61% reduction in lesion size respectively, compared to the water control, and a 38% and 43% reduction, respectively, when applied 7 d prior to inoculation (Figure 2A, B).
Figure 2. BioClay™ application provides a steady inhibition of Botrytis cinerea infection on tomato detached leaves.
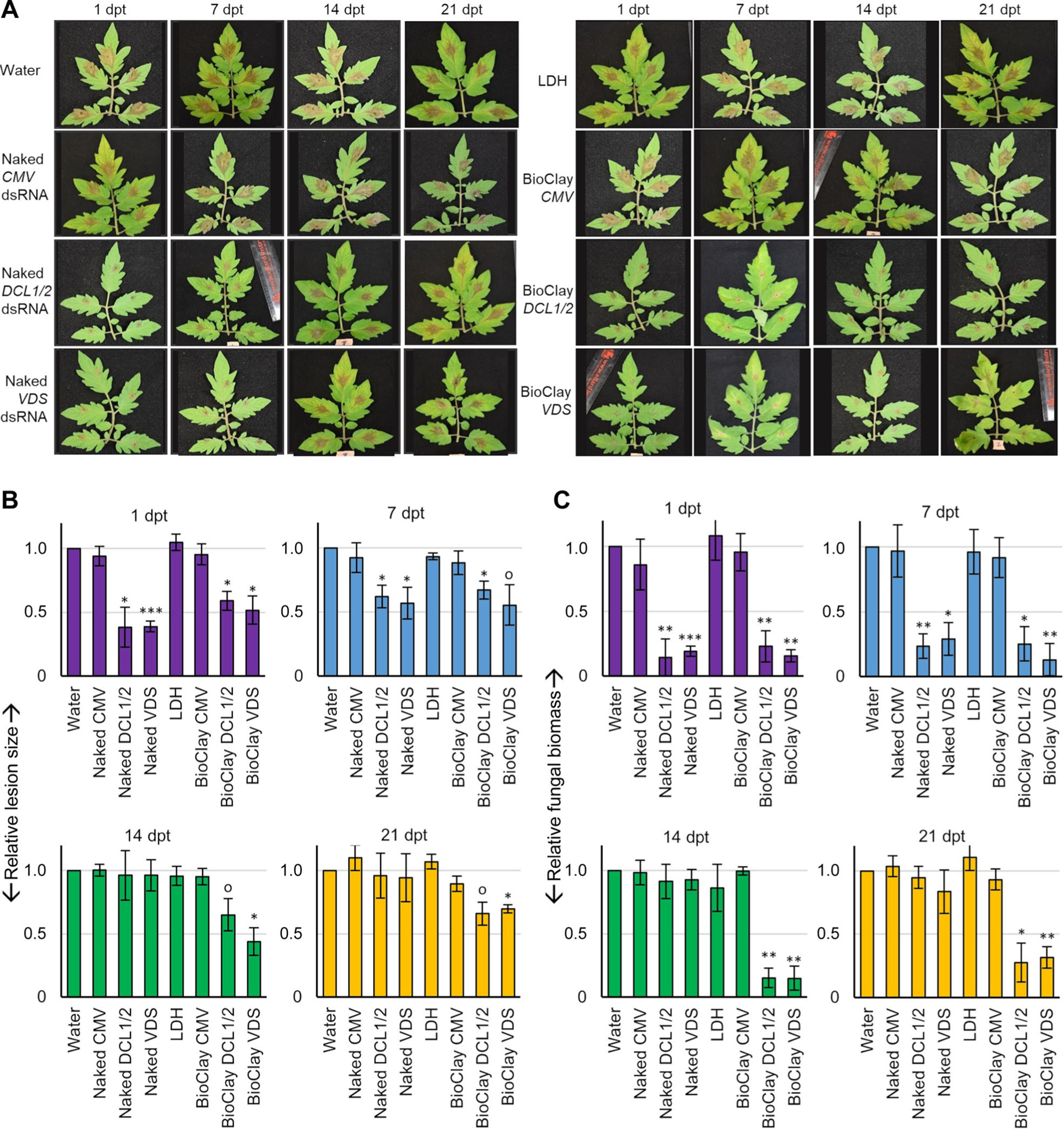
(A) Tomato detached leaves were pre-treated with water, naked dsRNA, LDH and BioClay for 1, 7, 14, and 21 d, then inoculated with B. cinerea spores. Pictures were taken at 4 d post inoculation. Representative inoculated detached leaves showed similar lesion sizes in the water control treatment, LDH treatment, CMV treatment (naked or BioClay) and both BcDCL1/2- and BcVDS-targeting naked dsRNA treatments at the later time points (14 and 21 d post treatment (dpt)). Smaller lesion sizes were observed at all time points with BioClay treatments (either BcDCL1/2 or BcVDS) and at the earlier time points (1 and 7 dpt) for the naked dsRNA treatments. (B) Relative lesion size areas were measured 4 d post inoculation on detached tomato leaves with the help of an electronic calibrator, assigning a value of 1.0 to the average lesion size area in the water treatment. (C) Relative fungal biomass was quantified by reverse transcription quantitative polymerase chain reaction (RT-qPCR) in detached leaves inoculated with B. cinerea. Fungal RNA relative to tomato RNA was measured with the fungal actin gene and the tomato actin gene by RT-qPCR using total RNA extracted from each time point (1, 7, 14, and 21 dpt). All measurements were referred to the value 1.0 obtained for the water treatment. Error bars indicate standard deviations (SD) obtained from four to seven biological replicates for the relative measurement of the lesion size area (B) and three biological replicates for relative quantification of fungal biomass (C). Levels of significant differences were determined by a one-way analysis of variance followed by Tukey’s HSD test and are indicated above the bars by (0P < 0.10; *P < 0.05; **P < 0.01; ***P < 0.001).
BcDCL1/2 BioClay application on the other hand significantly inhibited lesion development on tomato leaflets at all pre-treatment time points, with a 41%, 33%, 35%, and 34% reduction in lesion size compared to the water control for the 1, 7, 14, and 21 dpt, respectively (Figure 2A, B). BcVDS BioClay treatments also led to a prolonged effect compared to the naked BcVDS dsRNA. Lesion size reduction was 48%, 45%, 56%, and 30% compared to the water control at 1, 7, 14, and 21 dpt, respectively (Figure 2A, B).
To further investigate these findings, fungal biomass analysis was performed on the tomato leaflets. Overall, the biomass estimates reflected fungal lesion measurements on the tomato leaflets (Figure 2C). Specifically, there was an observable decrease in fungal biomass on the leaflets treated with naked dsRNA (BcDCL1/2 or BcVDS) at 1 dpt and 7 dpt compared to the water control, while at both 14 dpt and 21 dpt there was no difference in fungal biomass between the dsRNA and water-treated leaflets. Furthermore, the time between treatment and inoculation was found to be inversely related to fungal biomass, (BcDCL1/2: r = −0.85; P = 0.0004 and BcVDS: r = −0.83, P = 0.0009), i.e., the longer the time from treatment to fungal inoculation, the lower the protection. However, in the BioClay treatments, a significant decrease in fungal biomass (α < 0.05) in inoculated leaves was observed at all time points (Figure 2C), which was not correlated with the time between treatment and inoculation (BcDCL1/2 Bio-Clay: r = −0.04, P = 0.9 and BcVDS BioClay: r = −0.54, P = 0.07). Taken together, these results strongly suggest that BioClay provides protection with enhanced durability compared to naked dsRNA in detached leaf assays.
BioClay treatment reduces B. cinerea infection of mature chickpea plants
Thus far all BioClay formulations have been based on MgAl LDH. However, we recently showed that MgFe BioClay, which was developed as an alternative, is also an effective carrier of dsRNA (Jain et al., 2022). To test whether MgFe-based BioClay could provide similar protection against B. cinerea as MgAl-based BioClay, we looked at protection against B. cinerea in chickpea, a system of relevance to broadacre agriculture. Flowering is the most susceptible period of growth for chickpeas (Saxena and Johansen, 1997), therefore the assay was set up with mature plants. Six-week-old plants were treated 5 d prior to inoculation with either water, BcDCL1/2 dsRNA, BcDCL1/2 MgAl BioClay or BcDCL1/2 MgFe BioClay. Fourteen days after inoculation both the dsRNA and BioClay-treated plants were significantly protected from Botrytis infection (Figure 3). By 21 d, whilst naked dsRNA still provided some protection, the MgAl Bio-Clay treatments gave increased protection, with the MgFe BioClay trending towards being more protective than the naked dsRNA. By 28 d post inoculation, only the BioClay formulations were providing protection. Similar results were observed in an independent assay (Figure S1), with both MgAl and MgFe BioClay formulations providing statistically significant protection against B. cinerea at all time points after inoculation. Naked dsRNA was less effective in this repeated assay even at the earlier time points, highlighting that BioClay could provide durable protection for disease control well beyond what dsRNA alone may achieve in chickpeas. Together the observations made on detached tomato leaves and whole chickpea plants suggest that both MgAl and MgFe BioClay can provide a longer lasting protection against Botrytis infection than naked dsRNA, results consistent with previous findings in virus and insect pathosystems (Mitter et al., 2017; Jain et al., 2022).
Figure 3. BioClay™ provides protection against Botrytis on chickpea plants.
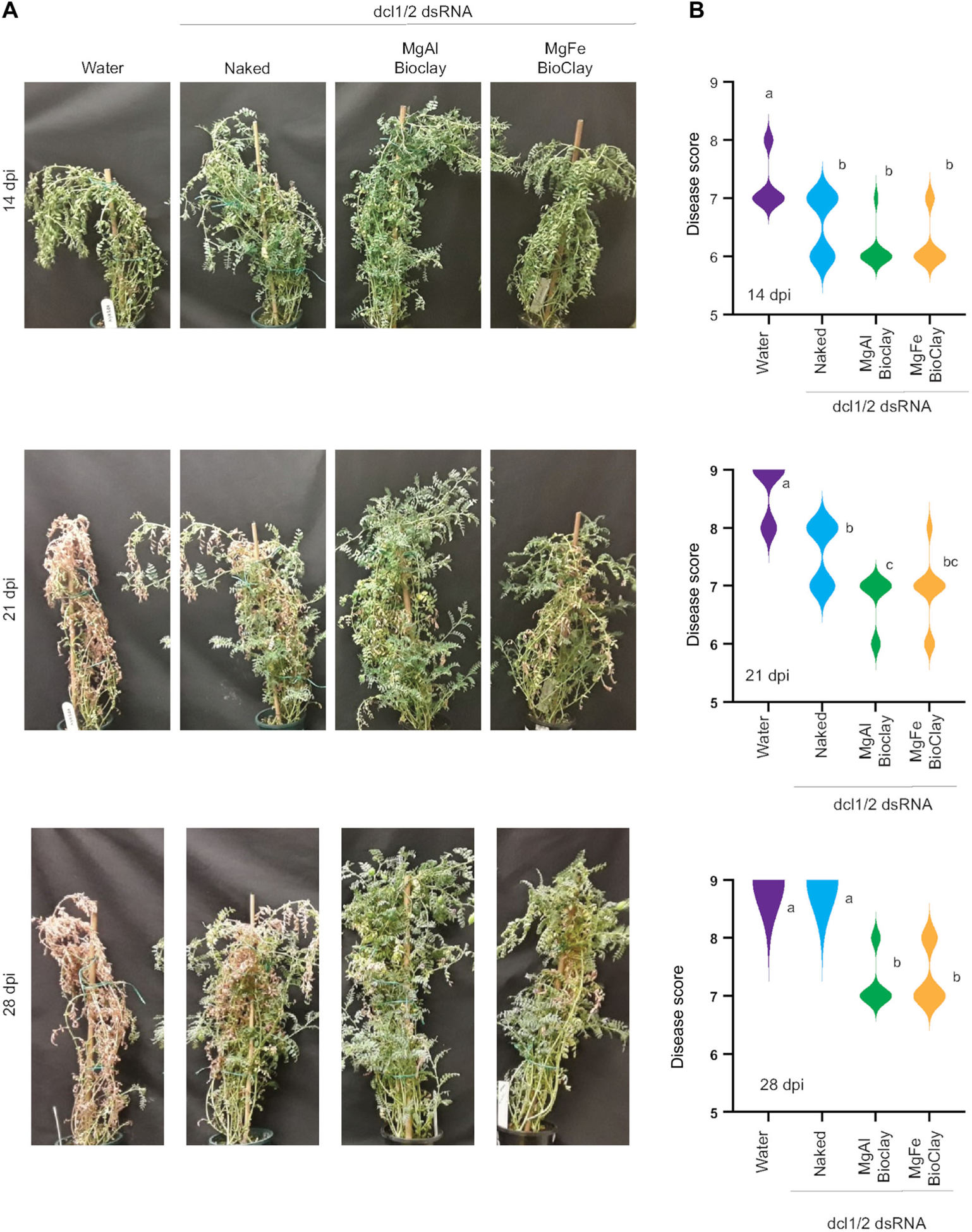
(A) representative images of chickpea plants infected with Botrytis with and without dsRNA or BioClay™ targeting BcDCL1/2. (B) Plant disease was scored on an ordinal scale from 1 to 9 and data analyzed using a Kruskal-Wallis test followed by Mann-Whitney tests for pairwise comparisons differences between treatments. Different letters indicate statistically significant (P < 0.05) groupings. Each treatment group consisted of nine individual plants.
BioClay treatment reduces post-harvest Botrytis gray mold disease symptoms
Next, we tested the ability of MgAl and MgFe BioClay to enhance post-harvest disease protection on tomato fruit. Similar to the leaf assays, treatment with BcDCL1/2 and BcVDS MgAl BioClay led to effective and long-lasting protection against B. cinerea disease development. Unlike previous experiments, treatments were applied as 20 μL drop-lets, and fruits were inoculated with fungal spores at 1, 5, and 10 dpt. Results were consistent with the assays conducted in tomato leaves. B. cinerea lesion size was only reduced in the naked dsRNA (both BcDCL1/2 and BcVDS) treatments when inoculation occurred 1 dpt, with reductions of 82% for BcDCL1/2 and 57% for BcVDS (Figure 4A, B). Fungal bio-mass assays revealed the same trend (BcDCL1/2: 87%; BcVDS: 90%) (Figure 4C). By 5 dpt there was no statistically significant reduction in B. cinerea lesion size, however, there was still a statistically significant reduction in fungal biomass (BcDCL1/2: 70%; BcVDS: 67%) (Figure 4C). The naked dsRNA treatments offered no protection against fungal disease at 10 dpt, and fungal biomass was found to be dependent on the time elapsed between treatment and application with naked dsRNA (BcDCL1/2: r = −0.90, P = 0.0008; BcVDS: r = −0.95, P = 0.0001). Application of MgAl LDH or BioClay containing a non-specific dsRNA sequence had no effect on B. cinerea lesion size or biomass (Figure 4). In contrast, treatment with BcDCL1/2 or BcVDS BioClay significantly reduced fungal lesion size at all three time points (BcDCL1/2 BioClay: 57%, 64% and 49% at 1, 5, and 10 dpt, respectively; BcVDS BioClay: 90%; 73% and 43%) (Figure 4A, B). The fungal biomass measurements supported these results, with no statistically significant dependence on the time between treatment and inoculation (BcDCL1/2 BioClay: r = −0.44, P = 0.2337; BcVDS BioClay: r = −0.53, P = 0.1401). Further, fungal biomass in fruit treated with MgAl BioClay never reached >25% of the fungal biomass of water treated fruit (Figure 4C).
Figure 4. BioClay™ application provides a steady inhibition of Botrytis cinerea infection on tomato fruit.
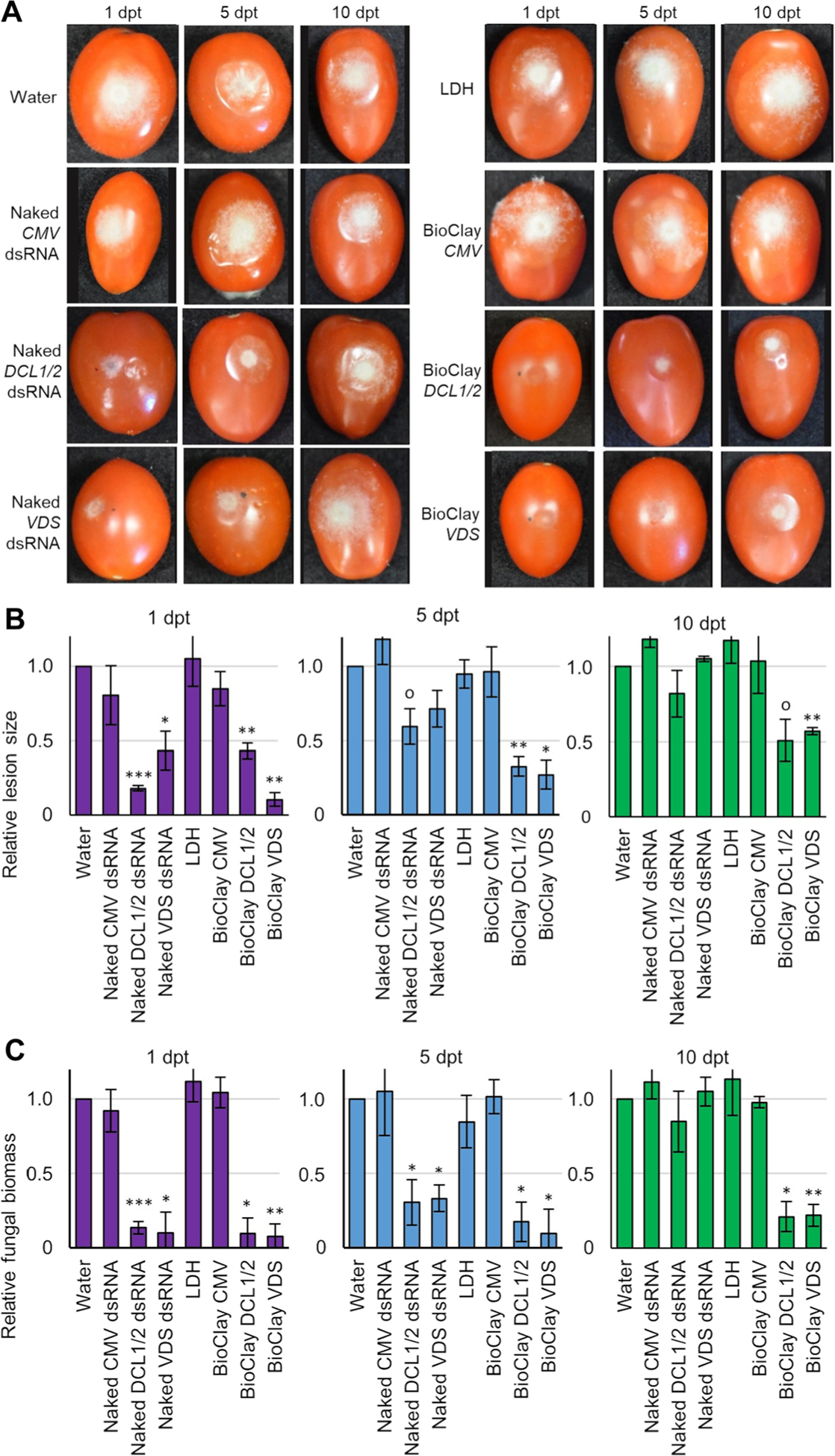
(A) Tomato fruit were treated with water, naked dsRNA, LDH or BioClay and were then inoculated with B. cinerea spores 1, 5, or 10 d post treatment (dpt). Pictures were taken at 6 d post inoculation. Representative inoculated tomato fruit showed similar lesion sizes in the water control treatment, LDH treatment, CMV treatment (naked or BioClay) and both BcDCL1/2- and BcVDS naked dsRNA treatments at the last time point (10 dpt). Smaller lesion sizes were observed at all time points with BioClay treatments (either BcDCL1/2 or BcVDS) and at 1 dpt in the naked dsRNA treatments. (B) Relative lesion size areas were measured 6 d post inoculation on tomato fruits with the help of an electronic calibrator, assigning a value of 1.0 to the average lesion size area in the water treatment. (C) Relative fungal biomass was quantified by reverse transcription quantitative polymerase chain reaction (RT-qPCR) in tomato fruits inoculated with B. cinerea. Fungal RNA relative to tomato RNA was measured with the fungal actin gene and the tomato tubulin gene by RT-qPCR using total RNA extracted from each time point (1, 5, 10 dpt). All measurements were referred to the value 1.0 obtained for the water treatment. Error bars indicate standard deviations (SD) obtained of at least three biological replicates. Levels of significant differences were determined by a one-way analysis of variance followed by Tukey’s HSD test and are indicated above the bars by (0P < 0.10; *P < 0.05; **P < 0.01; ***P < 0.001).
As with the MgAl-based formulations, treatment with BcDCL1/2 or BcVDS MgFe BioClay also resulted in a statistically significant decrease in B. cinerea lesion size (Figure 5A). BcDCL1/2 MgFe BioClay treatment resulted in a > 50% reduction in fungal growth at all time points (1, 5, and 10 dpt) and the BcVDS MgFe BioClay treatment resulted in a 48%–65% reduction (Figure 5B). Lesion size in MgFe BioClay treatments showed no statistically significant dependence on the time between treatment and inoculation (BcDCL1/2 MgFe BioClay: r = −0.51, P = 0.66; BcVDS MgFe BioClay: r = 0.9321, P = 0.24).
Figure 5. MgFe BioClay™ confers a steady inhibition of Botrytis cinerea infection on tomato fruit.
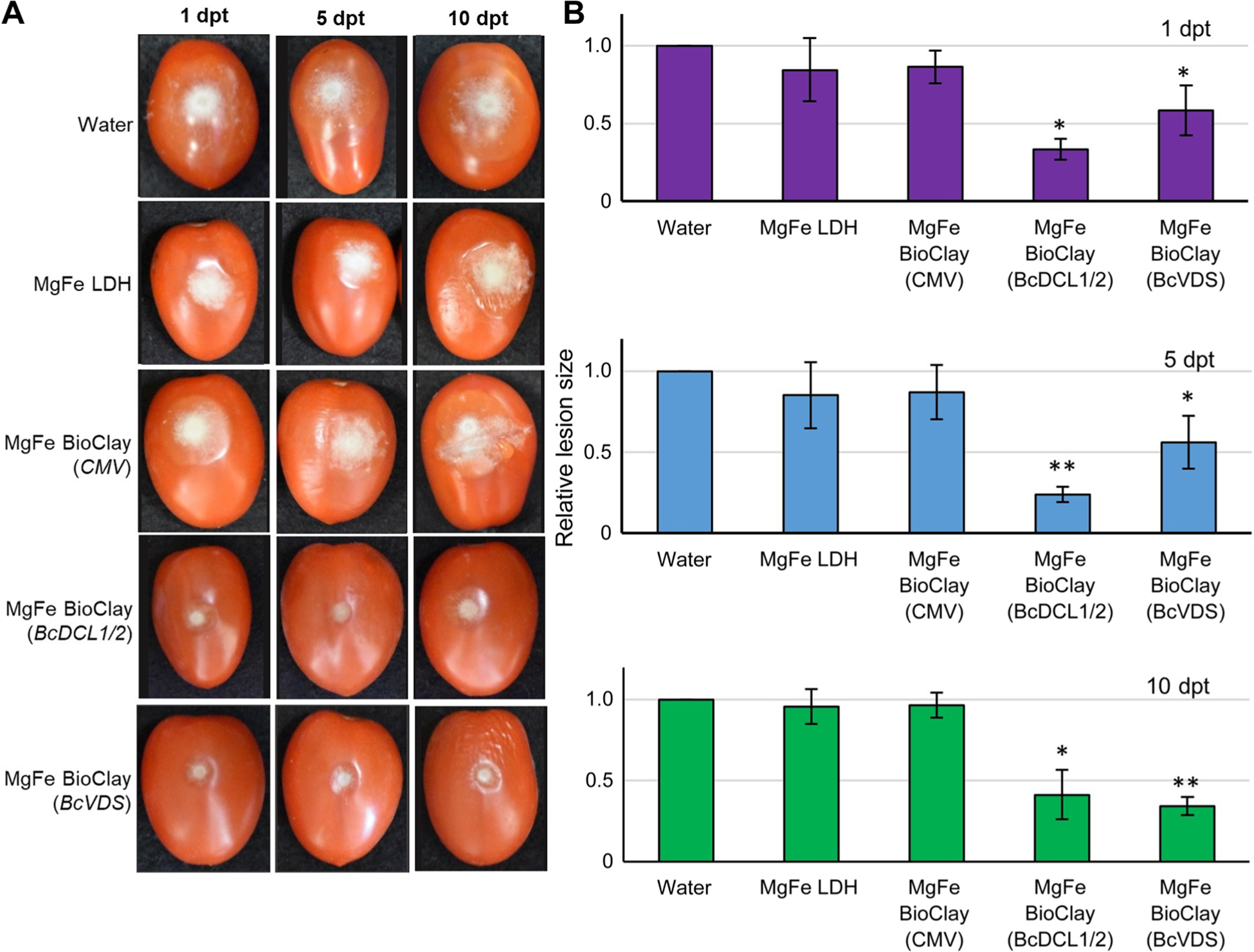
(A) Tomato fruit were pre-treated with water, MgFe-based LDH and MgFe-based BioClay™ for 1, 5, and 10 d, then inoculated with B. cinerea spores. Pictures were taken at 6 dpi. Representative inoculated tomato fruits showed similar lesion sizes in the water control treatment, LDH treatment, and CMV BioClay. Smaller lesion sizes were observed at all time points with BioClayTM treatments (either BcDCL1/2 or BcVDS). (B) Relative lesion size areas were measured 6 dpi on tomato fruits with the help of an electronic calibrator, assigning a value of 1.0 to the average lesion size area in the water treatment. Error bars indicate standard deviations (SD) obtained of at least three biological replicates. Levels of significant differences were determined by a one-way analysis of variance followed by Tukey’s HSD test and are indicated above the bars by (*P < 0.05; **P < 0.01).
DISCUSSION
SIGS constitutes an environmentally friendly approach for the control of fungal plant diseases. However, its commercial application is still limited due to the instability of dsRNA under field conditions (Cagliari et al., 2019; Dalakouras et al., 2020; Niño-Sánchez et al., 2021). This work investigated using LDH as an RNA carrier, extending on previous studies which have shown that LDH-bound dsRNA is protected from environmental degradation (Mitter et al., 2017; Jain et al., 2022).
We have demonstrated that B. cinerea can take up dsRNA released from BioClay, which is necessary for efficient SIGS (Qiao et al., 2021). Protection was observed in multiple systems following BioClay application, suggesting that dsRNA release is also occurring on plant surfaces. On plant surfaces, release of dsRNA from BioClay is expected to occur slowly as carbonic acid is formed from the dissolution of carbon dioxide in water films on the plant surface (Mitter et al., 2017; Jain et al., 2022). The observation that uptake by a fungal cell is dependent upon release suggests that BioClay sustains protection against fungal pathogens.
The application concentration of dsRNA is certainly an important factor for both the efficacy and the economic cost of the product application (Bocos-Asenjo et al., 2022). In these experiments, a 300 or 100 ng/μL concentration was used based on preliminary trials with different concentrations of dsRNA for the different Botrytis inoculations assays (data not shown) and previous studies carried out with BioClay (Mitter et al., 2017; Jain et al., 2022). This work reports the concentration which worked best, since excessive administration of dsRNA can produce non-specific effects due to saturation of the RNAi pathway and activation of the defense or immune pathways (Haller et al., 2019).
The extended protection afforded by BioClay compared to naked dsRNA indicates that the dynamics of dsRNA release facilitate sustained protection against Botrytis gray mold disease. Our results show that BioClay application not only hinders establishment of the fungus on pre- and post-harvest materials, but also that when the fungus becomes established in plant tissues, the development of the infection is much slower, which could allow the plant hosts to mature sufficiently to minimize losses due to disease. In addition, the observations made here with both MgAl- and MgFe-based BioClay suggest the extended protection in post-harvest assays is independent of the cationic species in the BioClay, offering considerable flexibility in the formulation to provide durable protection against Botrytis in tomato fruit and presumably against Botrytis in numerous other commodities both pre- and post-harvest.
This work extends the potential of BioClay technology from viruses and whiteflies (Mitter et al., 2017; Jain et al., 2022) to fungal pathogens. BioClay technology is currently being field-tested to allow its commercialization, with endless possibilities in regard to target genes (Rank and Koch, 2021).
MATERIALS AND METHODS
LDH synthesis and BioClay loading
MgAl and MgFe LDH were prepared as detailed in our previous work (Mitter et al., 2017; Jain et al., 2022). To determine the ratio at which naked dsRNA was completely complexed with the LDH, 300 ng of BcDCL1/2 or BcVDS dsRNA was combined with varying amounts of LDH (dsRNA: LDH from 1:1 to 1:5 (w/w)) and incubated at room temperature for 30 min. Complete loading of dsRNA onto LDH was confirmed when the dsRNA-LDH complex was retained in the well and unable to migrate through a 1% agarose gel. Following loading, sodium phosphate was added to the MgAl BioClay to a final concentration of 20 mM and ammonium sulphate was added to the MgFe BioClay to a final concentration of 10 mM.
dsRNA material
For pathology assays two dsRNA sequences were used. The first was based upon the B. cinerea dicer-like (DCL) genes, DCL1 (locus tag BCIN_12g06230) and DCL2 (locus tag BCIN_14g03910), originally described by Wang et al. (2016). The second construct, BcVDS, was based upon three genes originally identified as the targets of sRNAs shown to move from Arabidopsis to Botrytis (Cai et al., 2018b). These were vacuolar protein sorting 51 (VPS51, BCIN_16g04780), bilateral karyogamy defect 1 (bik1, BCIN_01g03260) required for the full functioning of the dynactin complex Bc-DCTN1 and suppressor of actin (SAC1, BCIN_14g01900) (Cai et al., 2018b; Qiao et al., 2021). The sequences are provided in Supplementary material. Both BcDCL1/2 and BcVDS dsRNA were synthesized by Genolutions (Korea) using their Agriculture Grade 2 service.
Fungal dsRNA uptake
Fluorescently-labeled YFP and DCL1/2 dsRNA was synthesized in vitro using the MEGAscript RNAi Kit (Life Technologies, Carlsbad, CA, USA) following the manufacturer’s instructions but with the addition of Fluorescein RNA labeling mix, a nucleotide mixture containing fluorescein-12-uridin-triphosphate (Sigma-Aldrich, St. Louis, MO, USA) for a final concentration of 1 mM NTP (including 0.35 mM fluorescein-12-UTP). Fluorescein-labeled YFP or DCL1/2 dsRNA (100 ng/μL) was added to germinated fungal spores on a glass microscope slide with 3 mL of PDA medium. The rapid release protocol used PDA media adjusted to pH 4 by addition of 10% citric buffer (50 mM, pH: 3), from the original pH 5.8. The mix was incubated at room temperature in the dark for 3 and 16 h and then treated with 1 μL micrococcal nuclease (MNase) (Thermo Fisher Scientific, Waltham, MA, USA) before analysis by confocal laser scanning microscopy (Leica TCS SP5, Lecia Microsystems, Wetzlar, Germany). Excitation was provided by an argon laser (488 nm) and the emission signal of fluorescein was detected at 495–545 nm.
Tomato protection assays
B. cinerea strain B05 was incubated on Malt Extract Agar (MEA) (Sigma-Aldrich, St. Louis, MO, USA) plates from frozen glycerol stock vials (−80°C). The fungus was incubated at 22°C for 10 d before spores were isolated from the plate using a sterile loop and incubated in 1% Difco™ Sabouraud Maltose Broth (BD Biosciences, Sparks, MD, USA). Spores were counted using a hematocytometer and spore concentration was adjusted in 1% Difco™ Sabouraud Maltose Broth.
For foliar inoculation of the tomato plants, commercial Money maker seeds were sown on soil and maintained at a controlled temperature (25°C) and high relative humidity (>70%) for 7–10 d until germination (relative humidity was stabilized at 70%). After 2 weeks, plants were transferred to 1-gallon pots with a mix of soil:sand (3:1 ratio). Plants were grown in a greenhouse with controlled temperature (25°C), and photoperiod (8-h night/16-h day) conditions, and were manually bottom watered every 2–4 d as needed. Tomato plants were incubated for another 3 weeks until three completely extended real leaves were present (identified as the first leaf with at least five leaf-lets). Afterwards, three real leaves from each plant were treated by spraying approximately 5 mL of a suspension of 100 ng/μL dsRNA or BioClay. We established four time points (1, 7, 14 and 21 d post treatment (dpt)) to harvest leaves and inoculate them with B. cinerea spores. Treated leaves were detached and placed into plastic boxes that were previously disinfected with a 10% bleach solution. Two pieces of wet filter paper were placed at the bottom of each box to maintain humidity. Each leaflet (five per leaf) was inoculated with a 10 μL drop of the B. cinerea spore suspension at 2×103 spores/mL.
On fresh local organic tomato fruits, the treatments were applied as 20 μL droplets of a solution with 100 ng/μL of dsRNA. Subsequently, tomato fruit were inoculated at 1, 5, and 10 dpt with 10 μL droplets of a B. cinerea spore suspension (106 spores/mL).
After inoculation, the detached leaves and tomato fruits were incubated for 4 and 6 d, respectively, inside the plastic box at 22°C with continuous light. Then, lesions were photographed to accurately determine size with the help of ImageJ software, and/or were measured by two perpendicular diameter measurements using an electronic calibrator OriginCal® Digital Caliper, (iGAGING, San Clemente, CA, USA). The diameter measurements were used to calculate the area of the ellipse contained in the lesion using the formula A = (D1 × 0.5) × (D2 × 0.5) × π.
Chickpea protection assays
Highly susceptible Kyabra chickpeas were grown in 15 cm diameter pots containing commercial grade potting mix (Richgro premium mix). Plants were grown and maintained at 22 ± 2°C under an 8-h night/16-h day photoperiod with a light that spans the wavelength from UV to far red light of 350–700 nm (Supplied by Valoya LED grow lights, Finland) at Griffith University, Nathan campus, Queensland, Australia. Bioassays were conducted in a completely randomized design in a controlled environment growth room with three replicates for each treatment × host combination with three plants per pot/replication (n = 9).
Simultaneously, a single-spored Australian B. cinerea isolate BC9653 was cultured on V8 juice agar plates and maintained at 22 ± 2°C with 12-h dark/12-h light periods provided by blue and cool white fluorescent lamps (400–700 nm) for 14 d. Subsequently, a spore suspension was prepared by harvesting pycnidiospores with sterile water, filtering through sterile cheesecloth (250 mm) and adjusting the final spore concentration to 1 × 105 spores/mL using a hemocytometer. Two to three drops of Tween 20 (0.02% v/v) were added per 100 mL of spore suspension as a surfactant. Six-weeks-old chickpea plants at flowering stage, were each sprayed with 5–6 mL of water, dsRNA or BioClay at 300 ng/μL. Five days later the plants were misted to run-off with a single spore inoculum and placed randomly in dark and humid sealed 20 L plastic crates (one crate/treatment). After 24 h post inoculation (hpi), crate lids were removed, and the plants were placed back into the 22 ± 2°C and 8-h night/16-h day growing conditions until disease assessment.
Each plant was assessed for disease severity by quantifying the percentage of plant infected by B. cinerea infection and a disease score between 1 and 9 was assigned to each plant using the scoring system of Anuradha et al. (2011) at 14, 21, and 28 d post inoculation (dpi). The scoring system is repeated here verbatim for clarity. 1 = no infection; 2 = minute water-soaked lesions on emerging tender leaves; 3 = minute water-soaked lesions on 1%–5% of leaves; 4 = water soaked lesions on 6%–10% of leaves and tender shoots; 5 = water-soaked lesions; soft rotting of 11%–25% of leaves and shoots; 6 = water-soaked lesions and soft rotting of 26%–40% of leaves and shoots; 7 = soft rotting and fungal growth on 41%–55% of the leaves and branches; 8 = soft rotting, fungal growth on 56%–70% of the leaves, branches, and stems; 9 = extensive soft rotting, fungal growth on above 70% of the leaves, branches and stems.
Data were analyzed in Prism 9.0 using a Kruskal-Wallis test to determine if there was any difference observed in the experiment based on treatments, which there were at all time points. This was followed by pairwise Mann-Whitney tests to determine pairwise differences between groups.
Total RNA extraction and reverse transcription quantitative polymerase chain reaction (RT-qPCR) for fungal biomass quantification
Samples of plant material inoculated with B. cinerea were collected and frozen at −80°C. Total RNA was extracted using TRIzol™ Reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. RNA was eluted in DEPC-treated water (Sigma-Aldrich, St. Louis, MO, USA), and treated with DNaseI according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA, USA). RNA integrity was tested using 1.2% agarose gels and quantified using a Nanodrop 2000c Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The absence of DNA was confirmed by the lack of conventional PCR amplification of tomato and Botrytis tubulin and actin genes, respectively. Then, cDNA was synthesized using the Superscript™ III First-Strand Synthesis System (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s instructions. The SYBR Green mix (Bio-Rad Laboratories, Hercules, CA, USA) was using to carry out RT-qPCR reactions in a CFX384 (Bio-Rad laboratories, Hercules, CA, USA) with the following thermal profile: 95°C for 15 min, followed by 40 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 30 s, and testing single amplification by a specific peak in the dissociation melting curve (0.5°C increments every 10 s from 65°C to 95°C). Fungal biomass in the inoculated samples was estimated as the relative quantity of actin transcript of B. cinerea (using Bc-actin-qRT-F: 5′-TGCTCCAGAAGCTTTGTTCCAA-3′ and Bc-actin-qRT-R: 5′-TCGGAGATACCTGGGTACATAG-3′ primers) normalized to the tubulin transcript (using Tomato-tubulin-F: 5′-GATTTGC-CCACTAACCTCTCGT-3′ and Tomato-tubulin-R: 5′-ACCT-CCTTTGTGCTCATCTTACCC-3′ primers of S. lycopersicum calculated by the 2−ΔΔCt method (Livak 2001).
Supplementary Material
ACKNOWLEDGEMENTS
This research was partially supported by the Australian Research Council Research Hub for Sustainable Crop Protection (IH190100022) funded by the Australian Government, and National Institute of Health (R35GM136379), National Science Foundation (IOS 2020731), United State Department of Agriculture (2021-67013-34258), and the CIFAR ‘Fungal Kingdom’ fellowship to H.J. J.N.S. was also supported by MINECO (PID2019-110459RB-I00) and MICINN (PLEC2021-008076) and A.S. was supported by an Advance Queensland Industry Research Fellowship.
Biographies
Jonatan Niño‐Sánchez

Hailing Jin

Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article: http://onlinelibrary.wiley.com/doi/10.1111/jipb.13353/suppinfo
REFERENCES
- Anuradha C, Gaur P, Pande S, Gali K, Ganesh M, Kumar J, and Varshney R (2011). Mapping QTL for resistance to botrytis grey mould in chickpea. Euphytica 182: 1–9. [Google Scholar]
- Bocos-Asenjo IT, Niño-Sánchez J, Ginésy M, and Diez JJ (2022). New insights on the integrated management of plant diseases by RNA strategies: Mycoviruses and RNA interference. Int. J. Mol. Sci 23: 9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagliari D, Dias NP, Galdeano DM, Dos Santos EÁ, Smagghe G, and Zotti MJ (2019). Management of pest insects and plant diseases by non-transformative RNAi. Front. Plant Sci 10: 1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, He B, Kogel KH, and Jin H (2018a). Cross-kingdom RNA trafficking and environmental RNAi — nature′s blueprint for modern crop protection strategies. Curr. Opin. Microbiol 46: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Qiao L, Wang M, He B, Lin F-M, Palmquist J, Huang S-D, and Jin H (2018b). Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 360: 1126–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, He B, Wang S, Fletcher S, Niu D, Mitter N, Birch PRJ, and Jin H (2021). Message in a bubble: Shuttling small RNAs and proteins between cells and interacting organisms using extracellular vesicles. Annu. Rev. Plant Biol 72: 497–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalakouras A, Wassenegger M, Dadami E, Ganopoulos I, Pappas ML, and Papadopoulou K (2020). Genetically modified organism-free RNA interference: exogenous application of RNA molecules in plants. Plant Physiol 182: 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schutter K, Taning CNT, Van Daele L, Van Damme EJ, Dubruel P, and Smagghe G (2022). RNAi-based biocontrol products: Market Status, regulatory aspects, and risk assessment. Front. Insect Sci 1: 818037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elad Y, Pertot I, Cotes Prado AM, and Stewart A (2016). Plant hosts of botrytis spp. In: Fillinger S and Elad Y eds. Botrytis – the Fungus, the Pathogen and its Management in Agricultural Systems, Springer International Publishing: Cham. pp. 413–486. [Google Scholar]
- European Commission (2019). Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions: The European Green Deal Document 52019DC0640.
- European Commission (2022). Proposal for a Regulation of the European Parliament and of the Council on the sustainable use of plant protection products and amending Regulation (EU) 2021/2115 Procedure 2022/0196/COD.
- Fisher MC, Hawkins NJ, Sanglard D, and Gurr SJ (2018). Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 360: 739–742. [DOI] [PubMed] [Google Scholar]
- Haller S, Widmer F, Siegfried BD, Zhuo X, and Romeis J (2019). Responses of two ladybird beetle species (Coleoptera: Coccinellidae) to dietary RNAi. Pest. Manag. Sci 75: 2652–2662. [DOI] [PubMed] [Google Scholar]
- He B, Cai Q, Qiao L, Huang CY, Wang S, Miao W, Ha T, Wang Y, and Jin H (2021). RNA-binding proteins contribute to small RNA loading in plant extracellular vesicles. Nat. Plants 7: 342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua L, Yong C, Zhanquan Z, Boqiang L, Guozheng Q, and Shiping T (2018). Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Qual. Saf 2: 111–119. [Google Scholar]
- Huang CY, Wang H, Hu P, Hamby R, and Jin H (2019). Small RNAs–big players in plant-microbe interactions. Cell Host Microbe 26: 173–182. [DOI] [PubMed] [Google Scholar]
- Huang Y, Wang S, Cai Q, and Jin H (2021). Effective methods for isolation and purification of extracellular vesicles from plants. J. Integr. Plant Biol 63: 2020–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RG, Fletcher SJ, Manzie N, Robinson KE, Li P, Lu E, Brosnan CA, Xu ZP, and Mitter N (2022). Foliar application of clay-delivered RNA interference for whitefly control. Nat. Plants 8: 535–548. [DOI] [PubMed] [Google Scholar]
- Koch A, Biedenkopf D, Furch A, Weber L, Rossbach O, Abdellatef E, Linicus L, Johannsmeier J, Jelonek L, Goesmann A, Cardoza V, McMillan J, Mentzel T, and Kogel K-H (2016). An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog 12: e1005901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A, and Kogel K-H (2014). New wind in the sails: Improving the agronomic value of crop plants through RNAi-mediated gene silencing. Plant Biotechnol. J 12: 821–831. [DOI] [PubMed] [Google Scholar]
- Li J, Li B, Wang J, He L, and Zhao Y (2021). Recent advances in layered double hydroxides and their derivatives for biomedical applications. Acta Chim. Sin 79: 238–256. [Google Scholar]
- Matthews A (2022). Implications of the European Green Deal for agri-food trade with developing countries. Brussels: European Landowners′ Organization [Google Scholar]
- McLoughlin AG, Wytinck N, Walker PL, Girard IJ, Rashid KY, de Kievit T, Fernando WGD, Whyard S, and Belmonte MF (2018). Identification and application of exogenous dsRNA confers plant protection against Sclerotinia sclerotiorum and Botrytis cinerea. Sci. Rep 8: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitter N, Worrall EA, Robinson KE, Li P, Jain RG, Taochy C, Fletcher SJ, Carroll BJ, Lu GQ, and Xu ZP (2017). Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 3: 16207. [DOI] [PubMed] [Google Scholar]
- Mosa MA, and Youssef K (2021). Topical delivery of host induced RNAi silencing by layered double hydroxide nanosheets: An efficient tool to decipher pathogenicity gene function of Fusarium crown and root rot in tomato. Physiol. Mol. Plant Pathol 115: 101684. [Google Scholar]
- Niño-Sánchez J, Chen LH, De Souza JT, Mosquera S, and Stergiopoulos I (2021). Targeted delivery of gene silencing in fungi using genetically engineered bacteria. J. Fungi 7: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu D, Hamby R, Niño-Sanchez J, Cai Q, Yan Q, and Jin H (2021). RNAs—A new frontier in crop protection. Curr. Opin. Biotechnol 70: 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrasch S, Knapp SJ, van Kan JAL, and Blanco-Ulate B (2019). Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol 20: 877–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Lan C, Capriotti L, Ah-Fong A, Niño-Sánchez J, Hamby R, Heller J, Zhao H, Glass NL, Judelson HS, Mezzetti B, Niu D, and Jin H (2021). Spray-induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake. Plant Biotechnol. J 19: 1756–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank AP, and Koch A (2021). Lab-to-field transition of RNA spray applications – How far are we? Front. Plant Sci 12: 755203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues T, Sridharan K, Manley B, Cunningham D, and Narva K (2021). Development of dsRNA as a sustainable bio-insecticide: From laboratory to field. Crop Prot. Prod. Sustain. Agric 17: 65–82. [Google Scholar]
- Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, and Nelson A (2019). The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol 3: 430–439. [DOI] [PubMed] [Google Scholar]
- Saxena NP, and Johansen C (1997). Integrated management of botrytis gray mold of chickpea: agronomic and physiological factors. In: Haware MP, Lenne JM, Gowda CLL eds. Recent Advances in Research on Botrytis Gray Mold of Chickpea, Patancheru: ICRISAT, pp. 21–28. [Google Scholar]
- Sun L, Wang J, Li L, and Xu ZP (2022). Dynamic nano-assemblies based on two-dimensional inorganic nanoparticles: Construction and preclinical demonstration. Adv. Drug Deliv. Rev 180: 114031. [DOI] [PubMed] [Google Scholar]
- Van de Wouw AP, Scanlan JL, Marcroft SJ, Smith AJ, Sheedy EM, Perndt NW, Harrison CE, Forsyth LM, and Idnurm A (2021). Fungicide sensitivity and resistance in the blackleg fungus, Leptosphaeria maculans, across canola growing regions in Australia. Crop Pasture. Sci 72: 994–1007. [Google Scholar]
- Wang M, Weiberg A, Lin F-M, Thomma BPHJ, Huang H-D, and Jin H (2016). Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. Plants 2: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiberg A, Wang M, Lin F-M, Zhao H, Zhang Z, Kaloshian I, Huang H-D, and Jin H (2013). Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 342: 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L-X, Hu J, Jia Y-B, Liu R-T, Cai T, and Ping Xu Z (2021). Two-dimensional layered double hydroxide nano-adjuvant: Recent progress and future direction. Nanoscale 13: 7533–7549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


