Abstract
Background:
The European Region, certified polio-free in 2002, remains at risk of wild poliovirus reintroduction and emergence of circulating vaccine-derived polioviruses (cVDPV) until global polio eradication is achieved, as demonstrated by the cVDPV1 outbreak in Ukraine in 2015.
Methods:
We reviewed epidemiologic, clinical and virology data on cVDPV cases, surveillance and immunization coverage data, and reports of outbreak-related surveys, country missions, and expert group meetings.
Results:
In Ukraine, 3-dose polio vaccine coverage declined from 91% in 2008 to 15% by mid-2015. In summer, 2015, two unrelated children from Zakarpattya province were paralyzed by a highly divergent cVDPV1. The isolates were 20 and 26 nucleotide divergent from prototype Sabin strain (with 18 identical mutations) consistent with their common origin and ~2-year evolution. Outbreak response recommendations developed with international partner support included conducting three nationwide supplementary immunization activities (SIAs) with tOPV, strengthening surveillance and implementing communication interventions. SIAs were conducted during October 2015-February 2016 (officially reported coverage, round 1–64.4%, round 2–71.7%, and round 3–80.7%). Substantial challenges to outbreak response included lack of high-level support, resistance to OPV use, low perceived risk of polio, widespread vaccine hesitancy, anti-vaccine media environment, economic crisis and military conflict. Communication activities improved caregiver awareness of polio and confidence in vaccination. Surveillance was enhanced but did not consistently meet applicable performance standards. Post-outbreak assessments concluded that cVDPV1 transmission in Ukraine has likely stopped following the response, but significant gaps in population immunity and surveillance remained.
Conclusions:
Chronic under-vaccination in Ukraine resulted in the accumulation of children susceptible to polioviruses and created favorable conditions for VDPV1 emergence and circulation, leading to the outbreak. Until programmatic gaps in immunization and surveillance are addressed, Ukraine will remain at high-risk for VDPV emergence and circulation, as well as at risk for other vaccine-preventable diseases.
Keywords: Poliomyelitis, Circulating vaccine-derived poliovirus, (cVDPV) outbreak, Supplementary immunization activities, (SIA), Polio outbreak response, WHO European Region, Ukraine
1. Background
The European Region of the World Health Organization (WHO) was certified free of poliomyelitis in 2002 [1]. However, until global polio eradication is achieved, the region remains at risk of wild poliovirus (WPV) reintroduction from endemic areas and emergence of circulating vaccine-derived polioviruses (cVDPV) in areas with suboptimal oral polio vaccine (OPV) coverage. These risks have been clearly demonstrated by poliovirus emergencies in Tajikistan in 2010 [2,3], Israel in 2013 [4], and most recently, in Ukraine where a highly divergent cVDPV type 1 (cVDPV1) paralyzed two children in 2015.
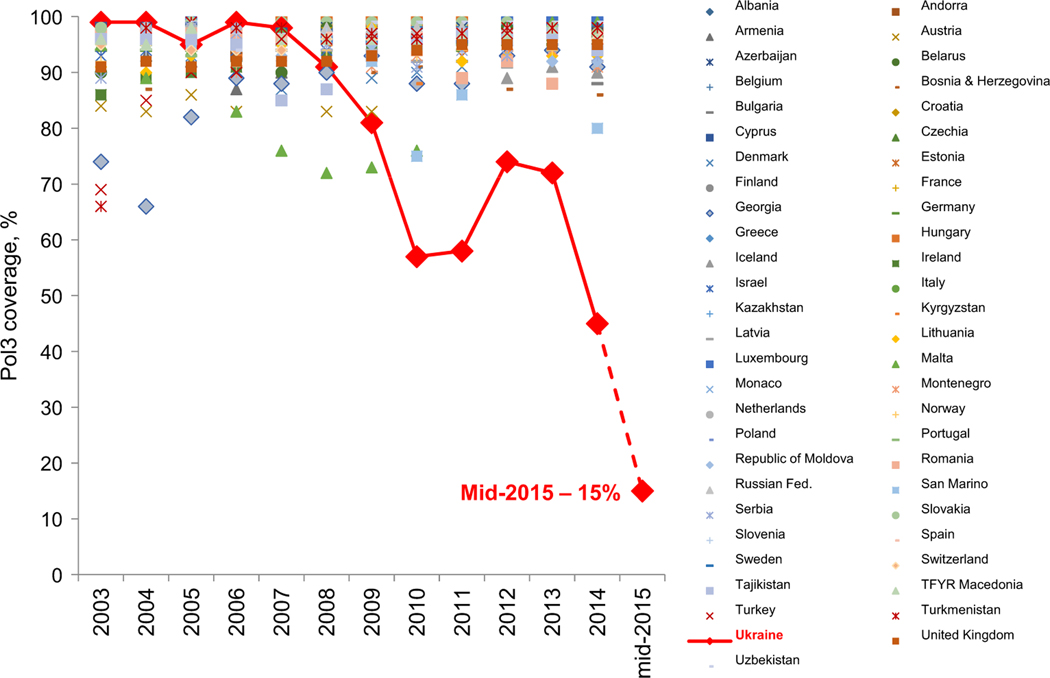
For decades, Ukraine3 had a well-developed immunization program with high coverage that allowed successful interruption of WPVs (Table 1). However, since 2009, there has been a sharp decline in coverage for all vaccines. Coverage with three doses of polio vaccine has declined to < 50% in 2014 and as low as 15% by mid-2015 (Fig. 1).
Table 1.
Timeline of polio-related events in Ukraine.
| Years | Events |
|---|---|
| 1960–2005 | Routine polio vaccination with tOPV; high coverage |
| 1996 | Last wild poliovirus case in Ukraine |
| 2002 | Ukraine certified free of poliovirus by the RCC along with other countries of the WHO European Region |
| Since 2005 | Routine polio vaccination according to sequential schedule (2 doses of IPV at 2 and 4 months, followed by tOPV at 6 and 18 months and 6 and 14 years); high coverage until 2009 |
| 2008 | Failed mass immunization campaign against measles and rubella |
| Since 2009 | Sharp decline in coverage with polio and other vaccines, decline in vaccine acceptance |
| Since 2010 | Ukraine ranked by the RCC as being at high risk of poliovirus spread if importation occurred |
| Since 2012 | Problems with vaccine supply due to inefficient budgeting and vaccine procurement for the National Immunization Program |
| Since 2014 | Economic and political crisis, military conflict with large numbers of displaced populations |
| 2014 | Poliovirus events: three isolated detections of VDPV2 (2 in Zakarpattya and one in Luhansk province) with no evidence of further circulation |
| 2015 | Polio outbreak: 2 polio cases due to highly divergent cVDPV1 in Zakarpattya |
| October 2015– February 2016 |
Three nationwide immunization rounds with tOPV implemented in response to the cVDPV1 outbreak |
| 2015 | Vaccine procurement legislation changed to allow for procurement via international agencies |
| 2016 | tOPV use in Ukraine discontinued from April 18, 2016 as part of the Global switch from tOPV to bivalent OPV (bOPV) in routine immunization program. |
Abbreviations: tOPV, trivalent oral polio vaccine; bOPV, bivalent oral polio vaccine; IPV, inactivated polio vaccine; WPV, wild poliovirus; VDPV, vaccine-derived poliovirus; cVDPV, circulating VDPV; RCC, European Regional Certification Commission for Polio Eradication; WHO, World Health Organization.
Fig. 1.
Reported coverage with three doses of polio vaccines (Pol3) in Ukraine and other countries of the European Region, 2003–2015. The 2003–2014 data are based on annual country reports (source – WHO web site: http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tscoveragepol3.html); the Ukraine data for 2015 are preliminary, as of June 2015 (source – the Ministry of Health of Ukraine).
Factors contributing to this dramatic decline in immunization coverage include:
Long-lasting negative impact of the failed measles-rubella vac-cination campaign in 2008, when the death of a child was incorrectly attributed to vaccine.
Hostile anti-vaccine media environment.
Low acceptance of immunization by caregivers and health careworkers (HCWs).
Widespread false contraindications to immunization.
Inefficient vaccine procurement legislation and practices.
Insufficient budgetary funding for health, including immuniza-tions, exacerbated by an economic and political crisis and ongoing military conflict since 2014.
Chronic under-vaccination resulted in the accumulation of a large number of children susceptible to polioviruses (estimated by WHO at 1.5–1.8 million in 2014). Since 2010, the European Regional Certification Commission (RCC) for Polio Eradication has ranked Ukraine at high risk of poliovirus spread [9].4 Protracted low coverage in an OPV-using country created the conditions for VDPV emergence and circulation. Three type 2 ambiguous VDPVs (aVDPV) were detected in Ukraine in 20145 heralding the potential of their future emergence and spread.
Since 2011, international partners repeatedly raised concerns regarding the polio risk in Ukraine and worked with the authorities to prepare for the imminent outbreak. In early 2015, the “Plan for Accelerated Routine Polio Vaccination” was jointly developed by WHO, UNICEF, and the Ukrainian Ministry of Health (MOH), with United States Agency for International Development support. Polio vaccines were procured by the Canadian Government and the launch of this plan was scheduled for September 2015. However, after confirmation of cVDPV1 in late August, outbreak response activities became the priority. In this report we review the epidemiologic and operational aspects of the cVDPV1 outbreak in Ukraine and its implications.
2. Methods
We reviewed the epidemiologic, clinical and virology data on cVDPV cases and the information on immunization coverage and polio surveillance [acute flaccid paralysis (AFP), enterovirus and environmental surveillance] in Ukraine.6 Data sources include annual country reports via WHO-UNICEF Joint Reporting Forms, expert mission reports on the outbreak investigation and response, reports of the post-campaign coverage survey, 3- and 6-month outbreak response assessment (OBRA) missions, the 30th Meeting of the RCC [13], the International Monitoring Board (IMB) meetings [14,15], and the Statement of the 10th meeting of the International Health Regulations (IHR) Emergency Committee (EC) [16]. Information on the immediate outcome of the public communication interventions was obtained from UNICEF knowledge, attitude and practice surveys7 and independent monitoring data collected during the outbreak response.
3. Results
3.1. Outbreak description
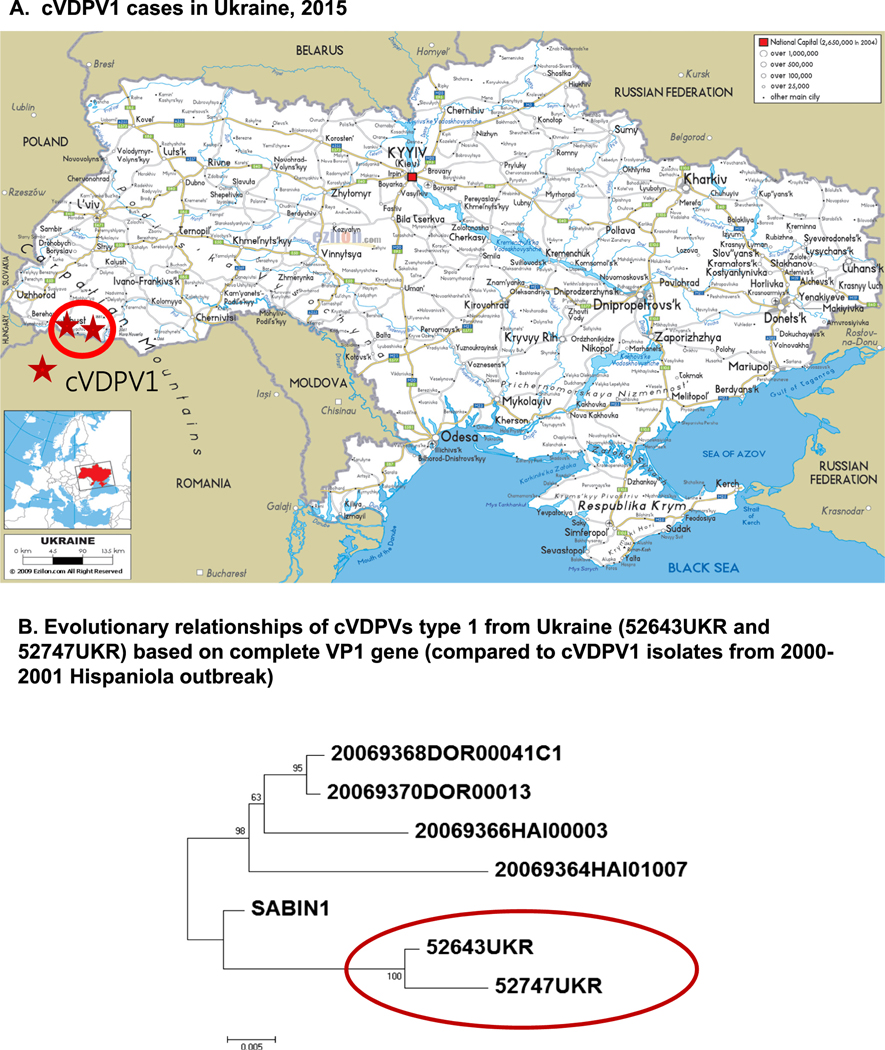
The outbreak was confirmed on August 28, 2015 when the WHO Polio Laboratory Network reported highly divergent cVDPV1 from two AFP cases from Ukraine. The case-patients, a 4 year-old and a 10 month-old unvaccinated children, had paralysis onset on June 30 and July 7, respectively. They were unrelated and lived in different districts of Zakarpattya province which borders Romania,8 Hungary, Poland, and Slovakia (Fig. 2).
Fig. 2.
Geographic location of cVDPV1 cases and phylogenetic relationships of the cVDPV1 isolates from Ukraine, 2015. The phylogeny was inferred using the Neighbor-Joining method [6]. The evolutionary distances were computed using the Tamura 3-parameter method [7] and are in the units of the number of base substitutions per site. Evolutionary analyses were conducted in MEGA7 [8].
Genomic sequencing revealed highly divergent vaccine viruses. The virus from one case had a 20 nucleotide (2.2%) difference from the prototype Sabin 1 strain, the virus from the other case had a 26 nucleotide (2.9%) difference. Eighteen of these mutations were shared by both viruses indicating their common origin and subsequent separate evolution (Fig. 2). The degree of genetic divergence from the parental strain is consistent with approximately 1.5–2.0 years of evolution before paralyzing these two children.
No additional cVDPV1 cases were identified through prospective enhanced surveillance. Retrospective active search for potentially missed AFP cases in the affected province during October-March period found no evidence of missed cases.
3.2. Outbreak response
On 1 September 2015 WHO informed Government of Ukraine of a polio outbreak due to cVDPV1. MOH publicly announced the outbreak immediately upon notification by WHO and initiated response activities with support of international experts (primarily from WHO and UNICEF).
To rapidly interrupt poliovirus transmission and in line with current internationally endorsed standards for responding to polio outbreaks [12], WHO and UNICEF recommended that the MOH:
Implement supplementary immunization activities (SIAs) consisting of three nationwide rounds using trivalent OPV (tOPV) with rounds 1 and 2 targeting all children aged < 6 years and round 3 with an expanded age range up to 10 years with a 1-month interval between rounds; the first round should begin within 14 days of the outbreak declaration.
Enhance poliovirus surveillance.
Implement comprehensive public communication interventionsto ensure public support for SIAs.
A large team of international experts was deployed to assist the MOH in the outbreak response, with the first arriving in Ukraine within 48 h of outbreak confirmation. Within two weeks, a national coordination mechanism was established, and an integrated evidence-based outbreak response strategy was drafted and approved by the Government and partners. International partners provided vaccines,9 technical, financial and logistical support and launched high level advocacy efforts for outbreak response. WHO and UNICEF led a large number of trainings building local capacity regarding the clinical and epidemiologic aspects of poliomyelitis, polio vaccines, polio eradication and outbreak response strategies, vaccine safety, vaccine management, surveillance, effective counselling, and communications. Approximately 9000 HCWs, 1400 educators, and 50 journalists were reached by these trainings.
In total, 42 international experts were deployed to Ukraine for various periods of time during September 2015-April 2016, accounting for 1910 working days in-country. This was in addition to the full involvement of WHO and UNICEF country offices and technical support provided through regular coordination calls and field visits by the regional and headquarter offices, as well as by US Centers for Disease Control and Prevention (CDC) and other partners.
3.3. SIAs
Although the MOH was committed to the outbreak response, substantial challenges to SIA implementation were posed by lack of visible high-level governmental support, denial of the outbreak by some politicians, clinicians and media outlets, and resistance by some prominent clinicians to OPV SIA in a country using IPV for the first two routine doses. Implementing a rapid, high-quality response was also complicated by a low perceived risk of polio paralysis, a strong fear of vaccine adverse events, an aggressive anti-vaccine media, groundless allegations about vaccine mishandling by MOH, and bureaucratic hurdles for vaccine importation and distribution. Additional difficulties were created by the humanitarian crisis and lack of working relations with areas currently outside the Government control.10 To mitigate these unique challenges, WHO and UNICEF acted as a “bridge” between the sides and asked the diplomats involved in the negotiations to encourage the local authorities to join the response efforts.
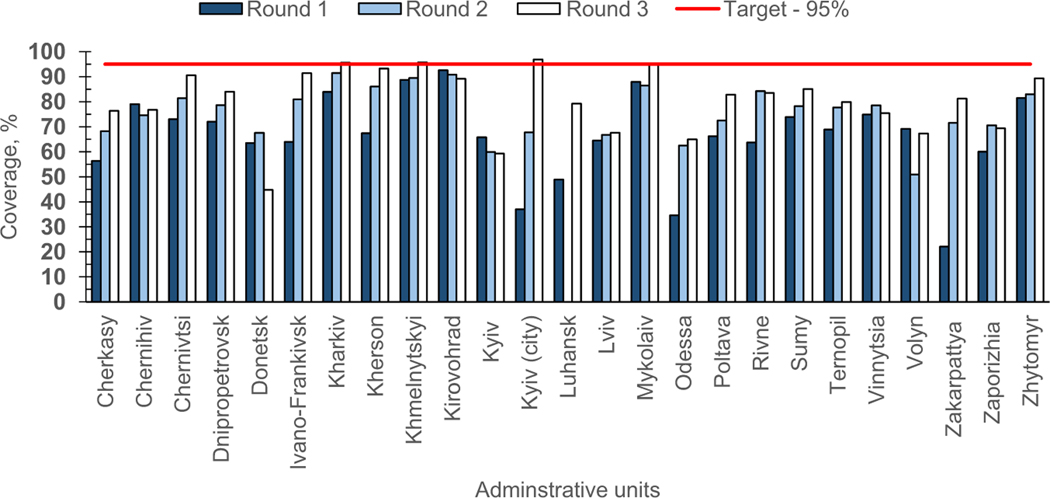
The SIAs with tOPV were initiated October 21 and implemented during October 2015-February 2016 (Table 2). SIA rounds 1 and 2 targeted 2.3 million children, and round 3, 3.7 million.11 Eligibility for SIA tOPV doses was dependent on prior vaccination with IPV - zero-dose children were given IPV during their first SIA contact.12 The coverage increased from 64.4% in round 1 to 80.7% in round 3. For each round, there was a substantial variation in coverage across provinces and few13 of them achieved the 95% target (Fig. 3).
Table 2.
Supplemental immunization activities (SIAs) in response to cVDPV1 outbreak – Ukraine, 2015–2016.
| Parameters | SIA dates | SIA type/vaccine used | Target age groups | No. targeted | No. vaccinated | Coverage reported,% | Coverage, post-campaign surveya, % |
|---|---|---|---|---|---|---|---|
| Coverage by SIA round as estimated by the Ministry of Health (MOH) and in a WHO-conducted post-campaign survey | |||||||
| SIA round 1 | 19 Oct-9 Nov 2015 | Natl./tOPV | 2 mos-5 yrs | 2,298,922 | 1,480,936 | 64.4 | 69.0 |
| SIA round 2 | 30 Nov-19 Dec 2015 | Natl./tOPV | 2 mos-5 yrs | 2,291,374 | 1,643,142 | 71.7 | 68.9 |
| SIA round 3 | 25 Jan-26 Feb 2016 | Natl./tOPV | 2 mos-9 yrs | 3,704,676 | 2,990,436 | 80.7 | 67.9b |
| Coverage by cohort of targeted children by the end of SIA round 3 | |||||||
|
| |||||||
| Age group | Per cent of children by number of vaccine doses received, as estimated by MOHc |
||||||
| 3 doses, % | 2 doses, % | 1 dose, % | 0 doses, % | Total, % | ≥1 dose, % | ≥2 doses, % | |
|
| |||||||
| 2 mos-9 yrs | 35.8 | 13.8 | 43.7 | 6.7 | 100 | 93.3 | 49.6 |
| 2 mos-5 yrs | 59.5 | 22.3 | 13.6 | 4.6 | 100 | 95.4 | 81.8 |
| 2 yrs | 62.0 | 20.8 | 13.5 | 3.7 | 100 | 96.3 | 82.8 |
| 3 yrs | 62.4 | 21.0 | 12.6 | 4.0 | 100 | 96.0 | 83.4 |
| 4 yrs | 63.3 | 20.6 | 12.2 | 3.9 | 100 | 96.1 | 83.9 |
| 5 yrs | 63.7 | 20.2 | 12.5 | 3.6 | 100 | 96.4 | 83.9 |
| 1 yrs | 58.5 | 24.2 | 14.2 | 3.1 | 100 | 96.9 | 82.7 |
| 2–11 mos | 43.6 | 29.2 | 17.7 | 9.5 | 100 | 90.5 | 72.8 |
| 6–9 yrs | 0.8 | 1.3 | 88.0 | 9.9 | 100 | 90.1 | 2.1 |
Abbreviations: Natl., national; N/A, not applicable; mos, months; yrs, years; tOPV, trivalent oral polio vaccine.
Post-campaign coverage survey implemented by WHO during February-March 2016.
Reflects coverage among children 2 months-5 years targeted by the WHO post-campaign survey.
Includes SIA tOPV doses as well as routine inactivated polio vaccine doses received during the SIA period.
Fig. 3.
Polio supplementary immunization activities (SIAs) in response to cVDPV outbreak: coverage by administrative unit and SIA round, Ukraine, 2015–2016.
Per MOH assessment, 95.4% of children aged <6 years received ≥1 vaccine dose (tOPV or IPV), but only 59.5% received all three doses during SIAs. Among 6–9 year-old children included in round 3, 90.1% received at least one dose of polio vaccine (Table 2). Post-campaign coverage survey results supported the official coverage reports (Table 2).
3.4. Strengthening surveillance
Ukraine has an established AFP and supplementary poliovirus (environmental and enterovirus) surveillance systems. Historically, AFP surveillance performance met the required standards (Table 3). To strengthen AFP surveillance during the outbreak period, the minimum target for non-polio AFP rate was raised from 2/100,000 recommended for high risk countries, to 3/100,000 recommended for outbreak countries [12]. In 2015, the national non-polio AFP rate was 2.67 with substantial variation at subnational levels. Six (24.0%) provinces14 met the 3/100,000 target rate, 11 (44.0%) achieved rates between 2 and 3/100,000,15 five (20.0%) provinces16 had non-polio AFP rate between 1 and 2/100,000, two provinces17 had a rate <1/100,000, and one province (Donetsk) reported no AFP cases. The comparison of immunization status of non-polio AFP cases during March-August 2015 versus September 2015-April 2016 showed an increase of the proportion of children aged <15 years that had received ≥3 doses of polio vaccines from 63% to 77%.
Table 3.
Performance of the acute flaccid paralysis (AFP) surveillance – Ukraine, 2003–2016.
| Variables | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016a | Target |
|---|---|---|---|---|---|---|---|---|---|---|
| Reported AFP cases, No. | 105 | 104 | 130 | 127 | 120 | 118 | 133 | 154 | 125 | N/A |
| Confirmed polio cases, No. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (cVDPV1–2, VAPP–1) | 0 | 0 |
| Polio-compatible cases, No. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Non-polio AFP rate | 1.67 | 1.70 | 2.10 | 2.05 | 2.01 | 2.09 | 2.18 | 2.67 | 1.97 | ≥1.0b |
| AFP surveillance indexc | 0.92 | 0.81 | 0.94 | 0.94 | 0.83 | 0.92 | 0.86 | 0.84 | 0.68 | ≥0.8 |
| AFP cases with 2 adequate stool specimens, %d | 95.6 | 86.6 | 94.4 | 93.9 | 87.5 | 94.4 | 89.1 | 86.3 | 82.7 | ≥80% |
2016 data are preliminary, as reported by week 35 2016; non-polio AFP rates and surveillance index for 2016 are annualized.
The target for non-polio AFP rate 2.0/100,000 applies to countries at high risk of poliovirus spread and 3.0/100,000 to countries with active polio outbreak.
Non-polio AFP rate up to 1.0 multiplied by the proportion of cases with stool specimen.
Per cent of AFP cases with 2 stool specimens collected 24–48 h apart within 14 days of onset.
The review of supplementary surveillance revealed an extensive laboratory network at province level, performing large numbers of tests on environmental and human samples, but with low yield due to often unclear criteria for environmental site selection and inconsistent laboratory testing practices. In 2015, a total of 11,062 specimens (316 from AFP cases, 6933 from humans, and 3777 from environmental surveillance) yielded 229 Sabin-like viruses (2.1%), and 130 (1.2%) NPEV, along with the cVDPV1 detected in stool samples from the 2 cases.
To increase the sensitivity and representativeness of supplemental surveillance, protocols were revised to ensure proper site selection for environmental specimen collection, optimize geographic areas and health care facilities for enterovirus surveillance, and improve laboratory testing methodology.
3.5. Communications and social mobilization
A comprehensive multi-channeled communication and social mobilization interventions aimed to increase public trust in vaccination and mitigate opposition to OPV use were led by UNICEF with WHO, Rotary, and other partners support. Caregivers with children aged <10 years were targeted nationwide (especially in poorly performing areas) through a carefully selected media mix using television, radio, outdoors, transit advertising, digital signage, social media, proactive media advocacy and events in busy areas. The communication campaign reached about 70% of Ukraine’s population. Special plans to reach high risk populations (Roma and internally displaced) were also executed.
The surveys found that the awareness of polio among caregivers increased from 68% at the beginning of the outbreak to 89% during SIA round 1, 91% during round 2, and 96% during round 3. The level of fear of vaccine-related complications among those parents who refused vaccination has decreased from 67% in 2014 to 38% by round 3. By round 3, 71% of caregivers believed that supplemental polio vaccination was needed.
3.6. Other activities
To mitigate the risk of international spread of VDPV1 from Ukraine, WHO conducted risk assessments and developed country-specific recommendations for countries bordering Ukraine. Additionally, a polio outbreak simulation exercise with participation from Czechia, Hungary, Republic of Moldova, Romania and Slovakia was held in October 2015 in Bucharest, Romania. Travel health notices related to polio in Ukraine were issued by WHO, CDC, European Center for Disease Control, and some countries.
3.7. Outbreak response assessments
To assess and guide the progress towards interruption of cVDPV1 transmission in Ukraine, outbreak response was repeatedly reviewed, including by the IMB (October 2015), Global Polio Eradication Initiative (GPEI) 3- and 6-month OBRAs led by WHO (December 2015 and April 2016), the RCC (June 2016), and the IHR EC (August 2016). The 6-month OBRA in April 2016 concluded and subsequent reviews concurred that cVDPV1 transmission in Ukraine has likely stopped. However, significant programmatic gaps in immunization and surveillance place Ukraine at the continued high-risk for VDPV emergence and circulation.
4. Discussion
As the world moves ever closer to global polio eradication, preventing the emergence and circulation of VDPVs becomes extremely important. Recognizing the risks posed by VDPVs, the GPEI Polio Eradication and Endgame Strategic Plan for 2013–2018 outlined an approach to eradicate WPV and eliminate cVDPVs in parallel [18]. In 2014, WHA declared the ongoing spread of polioviruses as a “public health emergency of international concern” [19,20]; and in 2015, the IHR EC for polio extended its Temporary Recommendations for WPV to cVDPV-affected countries [16,21].
Immunodeficiency-related and ambiguous VDPVs have been identified throughout the world, including the European Region, but all eight other cVDPV1 outbreaks known to date have occurred in economically underdeveloped tropical or subtropical settings [22]. Ukraine is the first country with a temperate climate, good sanitation, and generally well-developed, albeit problem-ridden health infrastructure [23,24] to document a cVDPV outbreak. The 2015 cVDPV1 outbreak was not an isolated occurrence. Detection of several aVDPV2s in different parts of Ukraine since 2014 indicates extensive population susceptibility and the potential for future VDPV emergence and circulation. Although only two clinical cases were identified, high infection to case ratio for polioviruses, genetic divergence, estimated time of evolution, and geographic spread of these cVDPVs indicate that hundreds of infections did occur [22,25].
The outbreak response planning in Ukraine was initiated within 72 h of notification, in compliance with internationally agreed standards [12]. However, because of the numerous challenges within and outside the health sector, progress in implementing outbreak response activities remained slow. The Government never declared the outbreak as a public health emergency – the standard for polio outbreaks in polio-free countries. The first SIA round did not start for nearly eight weeks, instead of the standard of within 14 days of case notification. None of the three nationwide SIA rounds achieved the 95% target. Nevertheless, >50% of children aged <6 years received three doses of polio vaccine during the outbreak response and there was an increase in the proportion of children vaccinated with ≥3 doses of polio vaccine among non-polio AFP cases reported before and after the outbreak confirmation. Improvement in population immunity following the SIAs was crucial evidence for the likely interruption of cVDPV1 transmission. The absence of major risk factors for poliovirus spread (high birth rate, high population density, poor sanitation and lack of health infrastructure) [26] in Ukraine, as well as potentially lower force of infection of VDPVs compared to WPV [22,25] were additional favorable factors for interrupting transmission.
A highly performing surveillance system is the key to the timely detection of polioviruses. AFP surveillance improved since the outbreak but has not consistently achieved the recommended enhanced rate. Variability in performance at subnational level and lack of information from the areas outside Government control raise concerns about insufficient sensitivity of AFP surveillance in some areas of Ukraine to detect poliovirus transmission. Lower than expected yield of NPEV and Sabin-like polioviruses (particularly during the SIAs) in environmental samples also suggests suboptimal sensitivity of supplementary surveillance. With completion of the global switch from tOPV to bOPV in April 2016 [27], the need for a highly sensitive, comprehensive polio surveillance system is critical as AFP surveillance will not detect non-paralytic infections with VDPVs.
The outbreak in Ukraine came as no surprise. Years of neglect resulted in the lowest immunization coverage in Europe and allowed the VDPV emergence and circulation. The outbreak highlighted the weakness of Ukraine’s immunization program and warned of the risk of outbreaks of other VPDs as well, if status quo is preserved. On a positive side, despite numerous shortcomings, the response to cVDPV1 by MOH was substantially different from the 2012 large scale outbreak of measles [28] – a disease targeted for elimination – that was allowed to burn itself out with more than 12,000 cases and no noticeable intervention.
The gradual round-to-round improvement in SIA coverage and increased community demand for polio vaccination and confidence in the immunization system during the outbreak response suggests that the outbreak may have been a turning point. For Ukraine, the response set a new standard for planning and delivering evidence-based immunization and communication strategies, and afforded an opportunity for improved coordination between stakeholders. Actions need to be taken now to ensure that this opportunity is not lost. The outbreak response activities generated a wealth of new knowledge and experiences that can serve as a solid foundation for reestablishing a high performing routine immunization program.
Due to gaps in routine immunization coverage, suboptimal coverage of the SIA rounds and the inability of surveillance to consistently achieve required standards, the 6 month-OBRA was cautious in concluding the interruption of cVDPV1 transmission in Ukraine and warned of continued risks. Despite being removed from the list of infected countries, the Temporary Recommendations still apply to Ukraine and progress reports to the RCC and IHR EC over the next two years will be needed to demonstrate continued progress in improving population immunity and surveillance quality [20].
To sustain gains made during the outbreak response and mitigate the risk of future VPD outbreaks in Ukraine, high-level political commitment will be crucial. Advocacy should continue to ensure that the appropriate legislative and regulatory frameworks for a fully functioning and adequately funded routine immunization program are in place. The adoption of legislation to procure vaccines through UNICEF beginning in 2016 is encouraging and should help ensure the supply chain with high quality vaccines at a reduced cost.
Ukraine received considerable support from international partners, which was instrumental in responding to the outbreak. Considering the current situation in Ukraine, efforts to improve polio immunization coverage and restore the routine immunization program will require substantial external support for the foreseeable future.
Despite improved population immunity gained from outbreak response activities, gaps in subnational coverage and a delay in the beginning of bOPV use for routine immunization leave Ukraine at continued risk for VDPV emergence. Ensuring uniformly high population immunity to all three types of polioviruses in Ukraine is urgent, especially after tOPV cessation in April 2016, which increases the risk for the VDPV2 emergence. A serosurvey to assess population immunity to polioviruses (and other VPDs) in Ukraine will help in evaluating the current level of population protection and developing strategies to further reduce susceptibility, including assessing the need for additional SIAs to address immunity gaps.
Acknowledgments
We would like to express our acknowledgement to the numerous staff and consultants participating in the outbreak response on behalf of the Ministry of Health of Ukraine, National Polio Laboratory of Ukraine, Regional Reference Laboratories in Helsinki and Moscow, WHO Country Office in Ukraine, WHO Regional Office for Europe, WHO headquarters in Geneva, CDC Office in Ukraine, Global Immunization Division and Polio and Picornavirus Laboratory at CDC in Atlanta, UNICEF/Ukraine, UNICEF/CEE, UNICEF Headquarters in New York, as well as Ukrainian frontline health care workers.
Funding
No additional funding for writing this report has been received.
Disclaimers
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry. Some of the co-authors are staff members of the World Health Organization (WHO). The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the WHO.
Footnotes
Population, 42.5 million (2016 estimate, State Statistics Service of Ukraine) [5]; area, 233,013 square miles; independent from the Soviet Union since 1991.
These viruses were unrelated and there was no evidence of their further circulation, therefore they were classified as aVDPVs and the episodes regarded as “poliovirus events” which do not require the same level of response as cVDPVs [12].
Crimea, currently not under Ukrainian Government control, was not included in the analysis.
Knowledge, attitude and practice surveys were conducted before, during and after the outbreak response (in 2014, 2015, and 2016). Generally, they followed similar protocols, but few questions in the 2014 baseline survey were different from the 2015 and 2016 surveys.
Since 2011, Romania is ranked by RCC as a country at high risk of poliovirus spread [17].
A total of 1.5 million doses of IPV and 12.6 million doses of tOPV funded by the Canadian International Development Agency (CIDA), CDC, and the European Community Humanitarian Aid Office (ECHO) has been supplied.
Parts of Donetsk and Luhansk provinces in Eastern Ukraine.
Children before the age of 2 months, the age when the 1st dose of polio vaccine can be given according to Ukrainian national immunization schedule, were considered ineligible for SIA.
IPV was provided by the partners prior to the outbreak in support of “The Plan for Accelerated Routine Polio Vaccination”. IPV administered during the SIA period was considered part of the routine immunization in accordance with Ukrainian national schedule. The recipients of the 1st dose of IPV were eligible to receive the SIA tOPV dose after 28 days.
Kharkiv, Khmelnytskiy, and Mykolayiv provinces and Kyiv City in round 3 only.
Zakarpattya, Lviv, Chernivtsi, Ternopil (all in western part of Ukraine), Kyiv and Zaporizhya provinces.
Chernihiv, Dnipropetrovsk, Ivano-Frankivsk, Kharkiv, Kirovohrad, Mykolayiv, Odesa, Rivne, Sumy, Vinnytsya and Volyn provinces.
Cherkasy, Kherson, Khmelnytskiy, Poltava provinces and City of Kyiv.
Luhansk and Zhytomyr.
Conflict of interest
None declared.
References
- [1].World Health Organization. Certification of poliomyelitis eradication. In: Fifteenth meeting of the European regional certification commission, Copenhagen, 19–21 June 2002. Copenhagen, Denmark: World Health Organization; 2005. p. 1–128. [Google Scholar]
- [2].Khetsuriani N, Pallansch MA, Jabirov S, et al. Population immunity to polioviruses in the context of a large-scale wild poliovirus type 1 outbreak in Tajikistan, 2010. Vaccine 2013;31:4911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Khetsuriani N, Pfeifer D, Deshevoi S, et al. Challenges of maintaining polio-free status of the European Region. J Infect Dis 2014;210(Suppl 1):S194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Anis E, Kopel E, Singer SR, et al. Insidious reintroduction of wild poliovirus into Israel, 2013. Eurosurveillance 2013;18:2–6. <http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20586>. [accessed 28 September 2016]. [DOI] [PubMed] [Google Scholar]
- [5].State Statistics Service of Ukraine. Population of Ukraine. <http://database.ukrcensus.gov.ua/PXWEB2007/eng/news/op_popul_e.asp> [accessed 26 November 2016].
- [6].Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. [DOI] [PubMed] [Google Scholar]
- [7].Tamura K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G + C-content biases. Mol Biol Evol 1992;9:678–87. [DOI] [PubMed] [Google Scholar]
- [8].Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 2016;33:1870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].World Health Organization. In: Report of the 23rd meeting of the European regional certification commission for poliomyelitis eradication. Copenhagen, Denmark, 28–29 June 2010. <http://www.who.int/immunization/sage/2_Polio_RCC23_Final_10_2010.pdf> [accessed 28 September 2016]. [Google Scholar]
- [10].World Health Organization. Report of the 21st meeting of the European regional certification commission for poliomyelitis eradication. Copenhagen, Denmark, 9–11 June 2008. <http://www.who.int/immunization/sage/5_EURO_Polio_RCC_Report.pdf>[accessed 28 September 2016]. [Google Scholar]
- [11].World Health Organization. Report of the 22nd meeting of the European regional certification commission for poliomyelitis eradication. Copenhagen, Denmark, 21–22 June 2009. <http://www.euro.who.int/__data/assets/pdf_file/0019/92017/E93603.pdf> [accessed 28 September 2016]. [Google Scholar]
- [12].Global Polio Eradication Initiative. Standard operating procedures. Responding to a poliovirus outbreak in a polio-free country. February 2015. World Health Organization, 2015. <http://www.polioeradication.org/Portals/0/Document/Resources/PolioEradicators/1a.PolioOutbreakGuideline20150220.pdf> [accessed 28 September 2016]. [Google Scholar]
- [13].World Health Organization. Report of the 30th meeting of the European regional certification commission for poliomyelitis eradication. Sarajevo, Bosnia and Herzegovina, 31 May-2 June 2016. <http://www.euro.who.int/__data/assets/pdf_file/0006/318651/Meeting-report-30th-RCC.pdf?ua=1> [accessed 28 September 2016]. [Google Scholar]
- [14].Independent monitoring board of the global polio eradication initiative. Twelfth Report, October, 2015 <http://www.polioeradication.org/Portals/0/Document/Aboutus/Governance/IMB/13IMBMeeting/13IMB_Report_EN.pdf> [accessed 28 September 2016].
- [15].Independent Monitoring Board of the Global Polio Eradication Initiative. Thirteenth Report, August, 2016. <http://www.polioeradication.org/Portals/0/Document/Aboutus/Governance/IMB/14IMBMeeting/14IMB_Report_EN.pdf> [accessed 28 September 2016].
- [16].World Health Organization. Statement on the 10th IHR Emergency Committee regarding the international spread of poliovirus, August 11, 2016. [accessed 28 September 2016]. [Google Scholar]
- [17].World Health Organization. Report of the 26th meeting of the European regional certification commission for poliomyelitis eradication. Copenhagen, Denmark, 18–20 June, 2012. <http://www.euro.who.int/__data/assets/pdf_file/0005/184739/e96806.pdf> [accessed 28 September 2016]. [Google Scholar]
- [18].Global Polio Eradication Initiative. Polio eradication and endgame strategic plan 2013–2018. <http://www.polioeradication.org/Portals/0/Document/Resources/StrategyWork/EndGameStratPlan_20130329_ENG.pdf> [accessed 28 September 2016].
- [19].World Health Organization. Statement on the meeting of the International Health Regulations Emergency Committee concerning the international spread of wild poliovirus, April 28–29 2014. <http://www.who.int/mediacentre/news/statements/2014/polio-20140505/en/> [accessed 28 September 2016].
- [20].World Health Organization. WHO Guidance for implementation of the IHR Temporary Recommendations under the IHR (2005) to reduce the international spread of polio. November 2015. <http://www.polioeradication.org/Portals/0/Document/Emergency/PolioPHEICguidance.pdf> [accessed 28 September 2016].
- [21].World Health Organization. Case definitions for the four diseases requiring notification to WHO in all circumstances under the IHR (2005). <http://www.who.int/ihr/survellance_response/case_definitions/en/> [accessed 28 September 2016].
- [22].Burns CC, Diop OM, Sutter RW, Kew OM. Vaccine-derived polioviruses. J Infect Dis 2014;210(Suppl 1):S283–93. [DOI] [PubMed] [Google Scholar]
- [23].Ukrainian Center for Social Reforms, State Statistical Committee of Ukraine, Ministry of Health of Ukraine, and Macro International Inc. 2008. Ukraine Demographic and Health Survey 2007. Calverton, Maryland, USA: UCSR and Macro International. <http://dhsprogram.com/pubs/pdf/FR210/FR210.pdf> [accessed 28 September 2016]. [Google Scholar]
- [24].Lekhan V, Rudiy V, Nolte E. Health care systems in transition: Ukraine. Copenhagen, WHO Regional Office for Europe on behalf of the European Observatory on Health Systems and Policies. <http://www.euro.who.int/__data/assets/pdf_file/0010/96418/E84927.pdf>; 2004 [accessed 28 September 2016].
- [25].Wringe A, Fine PE, Sutter RW, Kew OM. Estimating the extent of vaccine-derived poliovirus infection. PLoS ONE 2008;3:e3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sutter R, Kew O, Cochi S, Aylward B. Poliovirus vaccine – live. In: Plotkin S, Orenstein W, Offitt P, editors. Vaccines, 6th ed., Elsevier Saunders; 2013. p. 598–645. [Google Scholar]
- [27].Centers for Disease Control. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine — Worldwide, 2016. MMWR Wkly; 2016;65:934–8. [DOI] [PubMed] [Google Scholar]
- [28].World Health Organization Regional Office for Europe. Epidemiological Brief No. 29. <http://www.euro.who.int/__data/assets/pdf_file/0018/181800/EpiBrief-Issue-29.pdf?ua=1>; December, 2012 [accessed September 28, 2016].