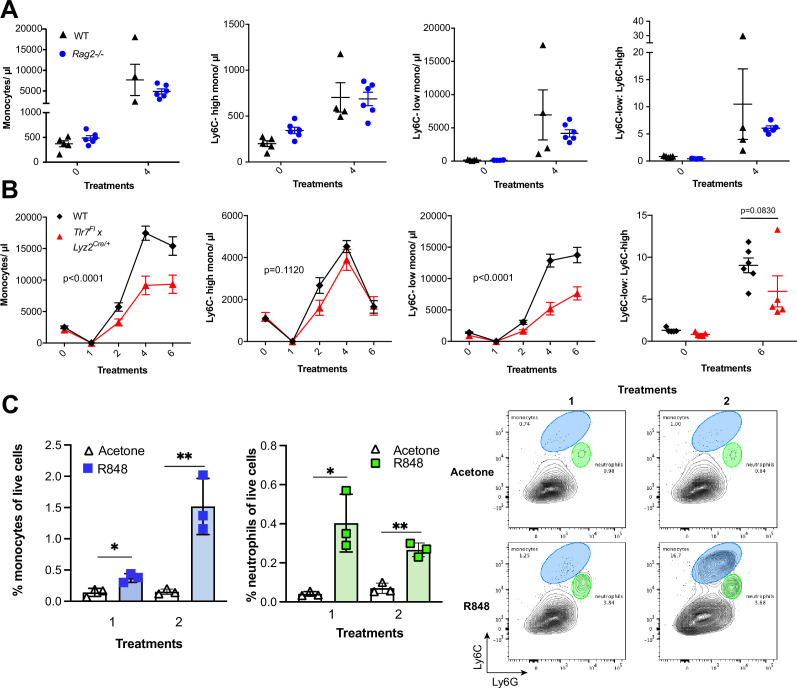
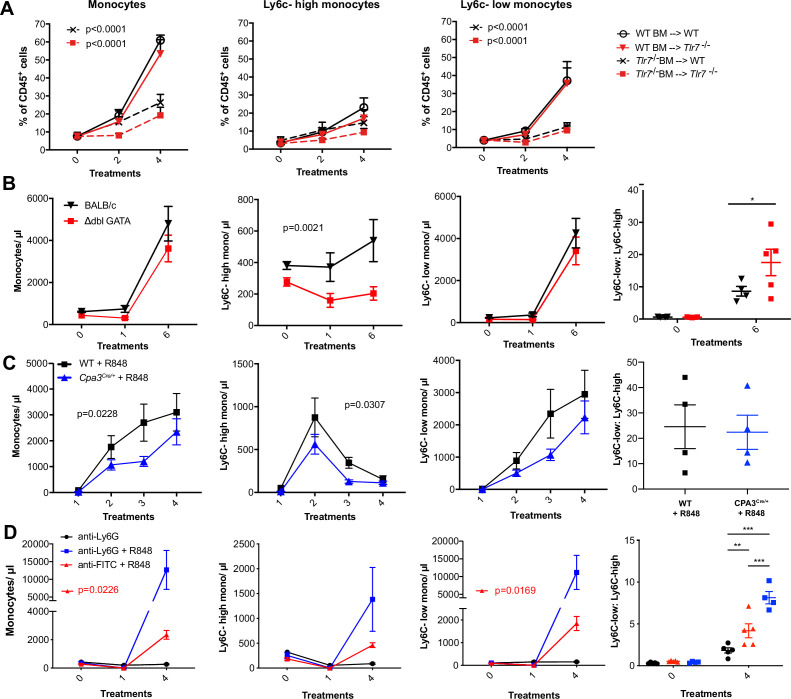
Figure 3. R848-induced monocytosis is driven by TLR7 activation of myeloid cells.
(A, B) Blood counts for total monocytes, Ly6C-high monocytes, Ly6C-low monocytes, monocyte subpopulation ratio, and lymphocytes at baseline and 24 hr after the indicated treatment. (A) C57BL/6 mice (n = 5, black triangles) and Rag2-/- mice (n = 6, blue circles) received four topical treatments with R848. (B) C57BL/6 mice (n = 6, black rhombi) and Tlr7fl × Lyz2Cre/+ mice (n = 5, red triangles) received six topical treatments with R848. (C) C57BL/6 mice (n = 3 per group) were treated once or twice with topical R848 or acetone. Treated ear skin was harvested at 24 hr post-treatment and anal- ysed by flow cytometry. Proportion of monocytes (CD11b+Ly6C+Ly6G-low) and neutrophils (CD11b+Ly6G-high- Ly6C-low) among total live cells (left panels). Representative flow cytometry plots gated on CD11b+ cells (right panels). Data represent a single experiment (A, C) or two experiments (B). Two-way ANOVA, with Tukey’s multiple-comparison for time-course experiments (A, B); unpaired t-test (C). Data are the mean ± SEM; only significant p-values are indicated; *p<0.05; **p<0.01.