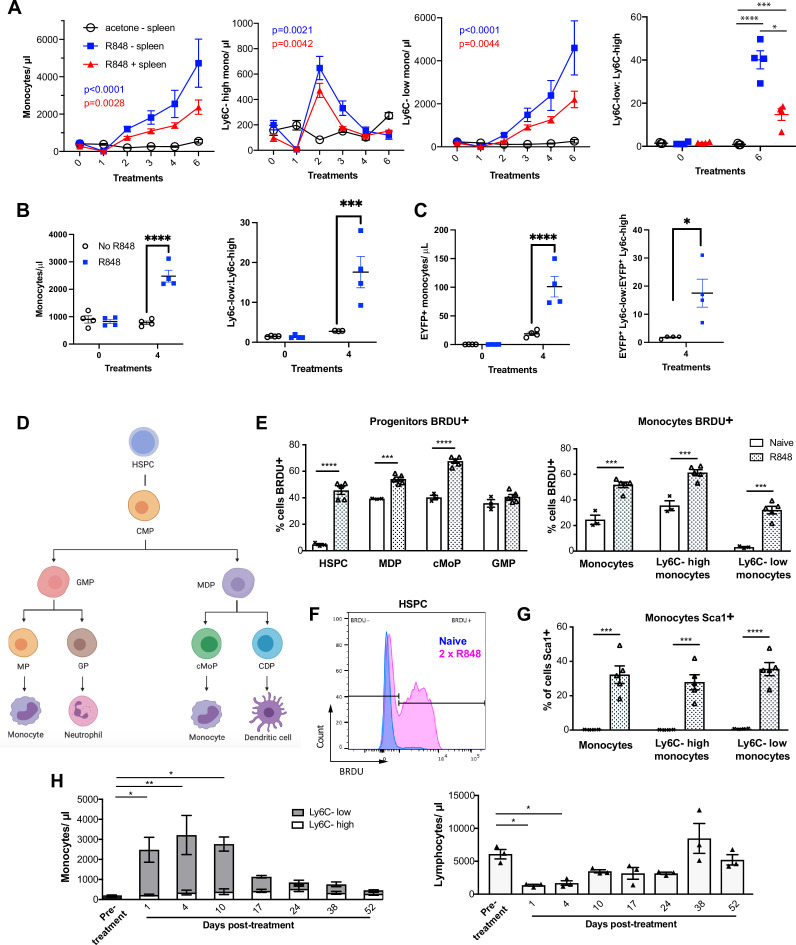
Figure 4. R848-induced monocytes are derived from the bone marrow (BM) and have features of emergency myelopoiesis.
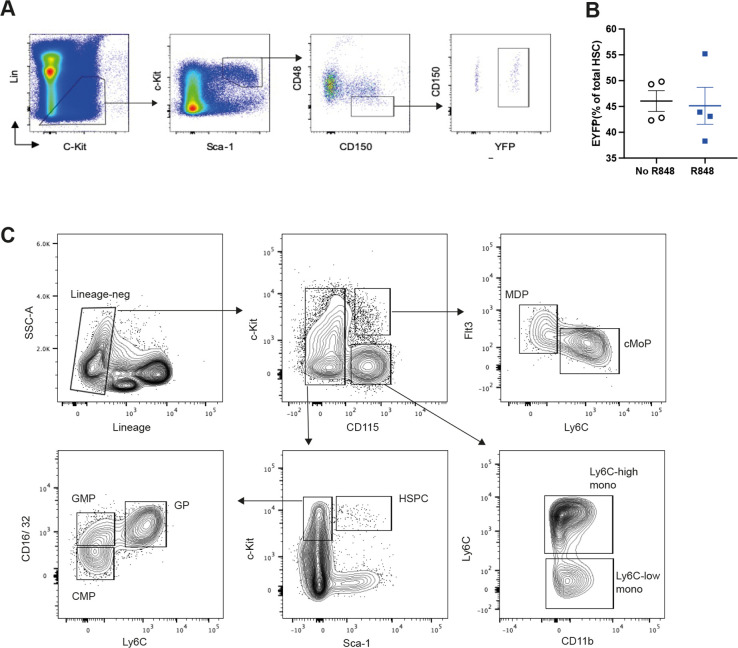
(A) BALB/c mice underwent splenectomy or sham surgery and were left to recover for 7 wk. Among the splenectomised mice, a group was treated 6× with topical R848 (n = 4, blue line) and the remaining mice with acetone (n = 3, solid black line). The sham surgery group was treated with R848 (n = 4, red line). Blood counts are shown for total monocytes, Ly6C-high monocytes, Ly6C-low monocytes, and the monocyte subpopulation ratio at baseline and 24 hr after the last treatments. (B, C) HSC-SCL-Cre-ERT;R26R-EYFP mice (n = 4 per group) received tamoxifen (4 mg/100 µl) by oral gavage for five consecutive days. Three days later, the left ears were treated topically 4× with R848 (blue squares) or left untreated (open circles). Shown are the total blood monocyte counts and the monocyte subpopulation ratio (B); the total blood EYFP+ monocyte counts and the subpopulation ratio among the EYFP-expressing monocytes at baseline and 24 hr after the last treatment (C). (D) Diagram illustrating BM myeloid progenitor differentiation: haematopoietic stem and progenitor cells (HSPC); common myeloid progenitor (CMP); monocyte-dendritic precursor (MDP); granulocyte-monocyte progenitor (GMP); common monocyte progenitor (cMoP); common dendritic progenitor (CDP); monocyte-committed progenitor (MP); granulocyte-committed progenitor (GP). (E–G) C57BL/6 mice were injected with 2 mg BRDU intraperitoneally (IP), either naïve (n = 3, white bars) or after two topical R848 treatments (n = 5, grey bars). Mice culled at 16 hr after the BRDU injection and BM harvested. (E) Percentage of BRDU positivity in HSPC, MDP, cMoP, GMP, total monocytes, Ly6C-high monocytes, and Ly6C-low monocytes. (F) Representative histogram of BRDU expression in HSPC at baseline (blue) or after 2× topical R848 (magenta). (G) Percentage of Sca1 positivity in total monocytes, Ly6C-high monocytes and Ly6C-low monocytes in the BM. (H) BALB/c mice (n = 3) received four treatments with topical R848. Blood counts for Ly6C-high monocytes (white bars), Ly6C-low monocytes (grey bars), and lymphocytes were monitored at the indicated time points after the cessation of treatment. Data representative of two independent experiments (except A and H). Two-way ANOVA with Tukey’s multiple comparison for time-course experiments (A–C, H); unpaired t-test (E, G). Data are the mean ± SEM; only significant p-values are indicated; *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.