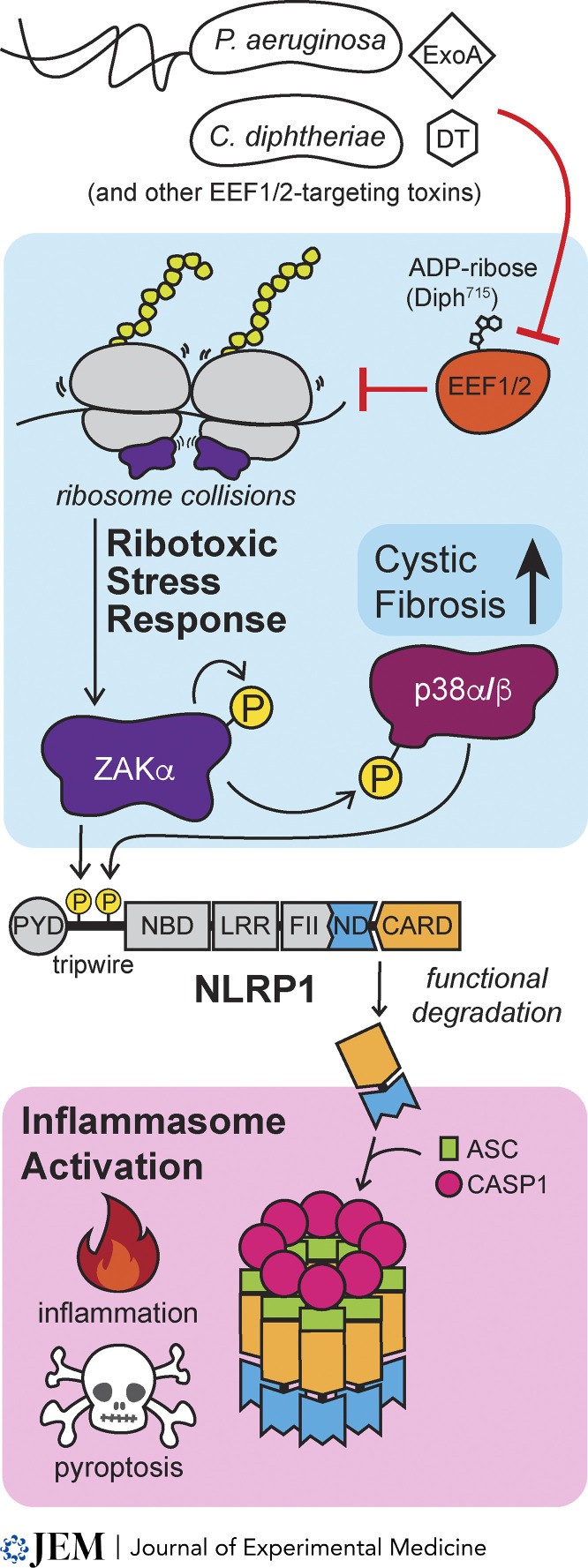
NLRP1 recognizes P. aeruginosa and C. diphtheriae through the RSR. DT and ExoA exotoxins ADP-ribosylate eukaryotic translation elongation factor 2 (EEF2) to inhibit protein synthesis and induce ribosome collisions that activate the MAP kinase kinase kinase ZAKα, initiating a signaling cascade culminating in p38α and p38β phosphorylation. Both ZAKα and p38α/β phosphorylate NLRP1’s N-terminal “tripwire,” leading to its functional degradation. The bioactive C terminus of NLRP1 complexes with ASC and CASP1 to form an active inflammasome and drive subsequent inflammation and pyroptotic cell death.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.