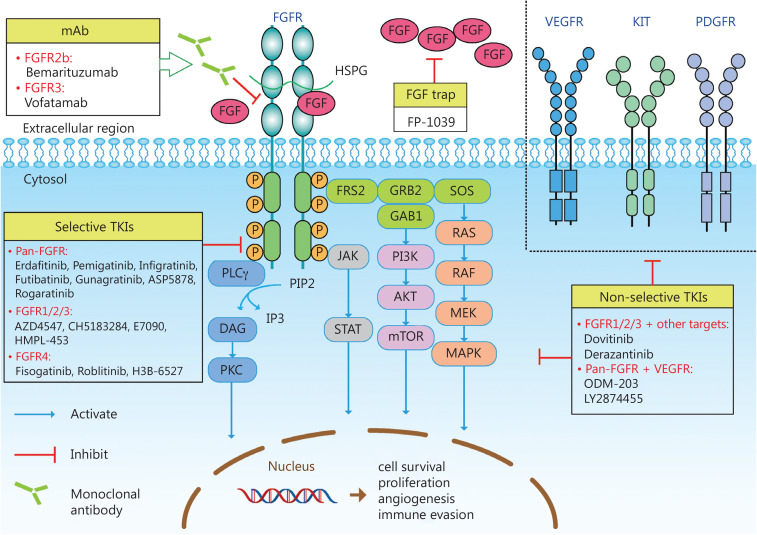
Figure 1.
FGFR signaling and inhibitors. The family of FGF receptors (FGFR1-4) are receptor tyrosine kinases expressed on cell membranes with significant sequence homology. Each FGFR typically consists of three extracellular immunoglobulin-like domains, a hydrophobic transmembrane domain, and two intracellular tyrosine kinase (TK) domains. FGF and FGFR binding stimulates receptor dimerization. This interaction can be stabilized by heparan sulfate proteoglycans (HSPGs). FGF-FGFR binding further phosphorylates intracellular FGFR substrate 2 (FRS2), phospholipase C gamma (PLCγ), and JAK, thereby activating four major signaling pathways. (1) The activation of FRS2 recruits the adaptor proteins [GRB2 and son of sevenless (SOS)], which results in subsequent activation of MAPK. (2) GRB2 recruits GAB1, which leads to activation of the PI3K-AKT-mTOR pathway. (3) Phosphorylation of PLCγ hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-tri-phosphate (IP3) and diacylglycerol (DAG), thus activating protein kinase C (PKC). (4) JAK-STAT signaling can also be activated. FGF/FGFR pathway inhibitors are mainly divided into mAb/FGF trap, which prevent FGF and FGFR binding in the extracellular domain, and small molecule TK inhibitors (TKIs) that target the ATP-binding cleft of TK domains inside the cell. Selective TKIs specifically target the FGFR kinase domains, while non-selective TKIs target several phylogenetically-related growth factor receptors, such as VEGFR, KIT, and PDGFR. JAK, Janus kinase; STAT, signal transducer and activator of transcription; GRB2, growth factor receptor-bound protein 2; GAB1, GRB2-associated binding protein 1; VEFGR, vascular endothelial growth factor receptor; PDFGR, platelet-derived growth factor receptor.