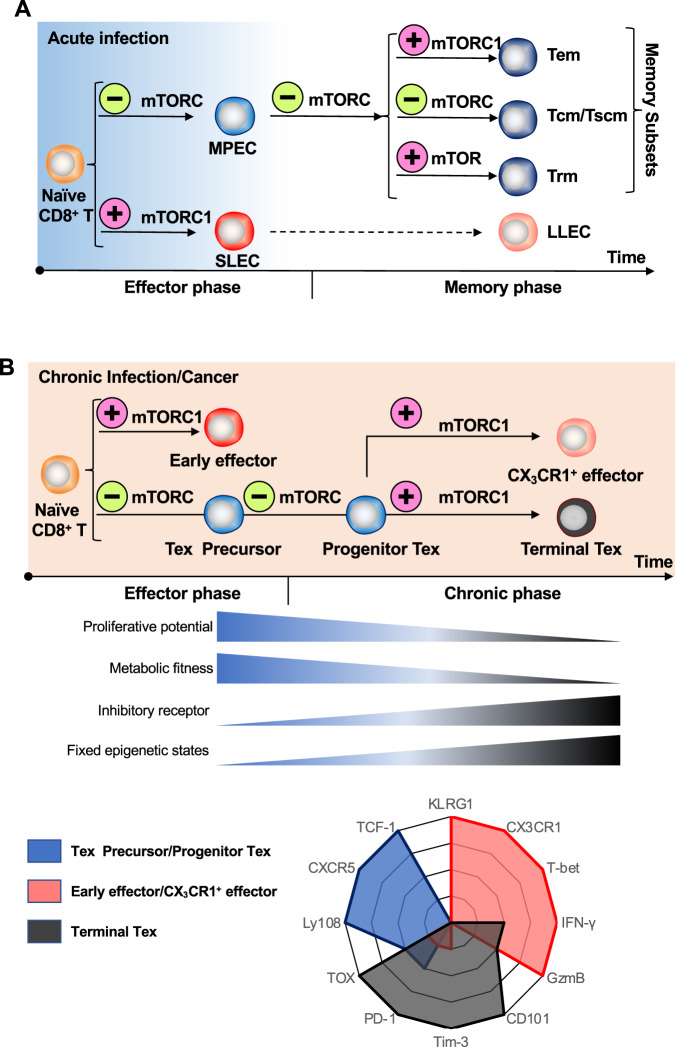
Fig. 2.
The role of the mTOR signaling pathway in the differentiation of memory/exhaustion T-cell subsets. A During acute infection, virus-specific naïve CD8+ T cells differentiate into SLECs that display potent cytotoxicity and MPECs that have a greater capacity to form memory cells following viral clearance. After the antigen has been eliminated, a minority of SLECs manage to survive and transform into LLECs. On the other hand, MPECs develop into various types of memory T cells, including Tem, Tcm, and Trm. mTORC1 activity instructs SLEC and Tem differentiation. Inhibition of mTOR, either by Rapamycin treatment or through siRNA-mediated knockdown, promotes MPEC and memory formation, particularly for Tcm and human Tscm. However, mTOR activation in T cells promotes their differentiation into Trm cells and enhances their survival in peripheral tissues. B During chronic infection or cancer, virus-specific naïve CD8+ T cells are activated and segregate into early effector cells and precursor cells. The precursor cells develop into Progenitor Tex, which further differentiate into terminally exhausted cells and effector-like cells marked by CX3CR1 expression. Inhibition of mTOR activity enhances Progenitor Tex formation at both the early and late stages. However, Progenitor Tex cells retain the ability to activate the mTOR pathway in response to antigen receptor signals, and mTOR is required for the transition of Progenitor Tex cells into exhausted and effector cells in the chronic phase. MPEC memory precursor effector cells, SLEC short-lived effector cells, LLEC long-lived effector cells, Tem effector memory T-cell, Tcm central memory T cells, Tscm T memory stem cells, Trm resident memory T cells, Progenitor Tex progenitor exhausted T-cell