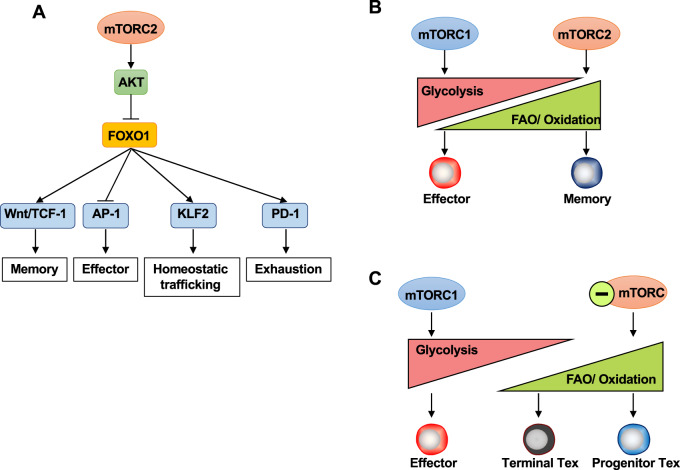
Fig. 4.
The role of the mTOR signaling pathway in metabolic programs and differentiation of CD8 T-cell subsets. A The mTORC2-Akt pathway promotes FOXO1 phosphorylation, resulting in decreased nuclear accumulation of FOXO1. Nuclear FOXO1 promotes memory formation through the Wnt/TCF1 pathways, directly binds and suppresses AP-1 transcription factors that are known to be key regulators of effector programs, induces KLF2 expression to regulate homeostatic trafficking, and sustains PD-1 expression while inducing the terminal differentiation of exhausted CD8+ T cells during chronic infection. B Naïve T cells uptake low levels of glucose and amino acids and rely on mitochondrial oxidative phosphorylation (OXPHOS). Upon T-cell activation, effector CD8+ T cells require high levels of glucose metabolism to support their rapid proliferation and production of cytokines and cytotoxic molecules. During an immune response, effector cells undergo a metabolic switch from OXPHOS to glycolysis. In contrast, memory CD8+ T cells have a more quiescent metabolism and rely more on OXPHOS for energy production. Memory cells also exhibit higher levels of fatty acid oxidation. mTORC1 activity is required to sustain high levels of glycolysis in effector T cells in both acute and chronic infections. Inhibition of mTORC2 activity, on the other hand, enhances the metabolic capacity of CD8+ T cells. C Tex have been reported to exhibit metabolic insufficiency with suppressed oxidation and glycolysis. Early progenitor Tex cells exhibit and retain a catabolic metabolism characterized by mitochondrial fatty acid oxidation (FAO) and oxidation