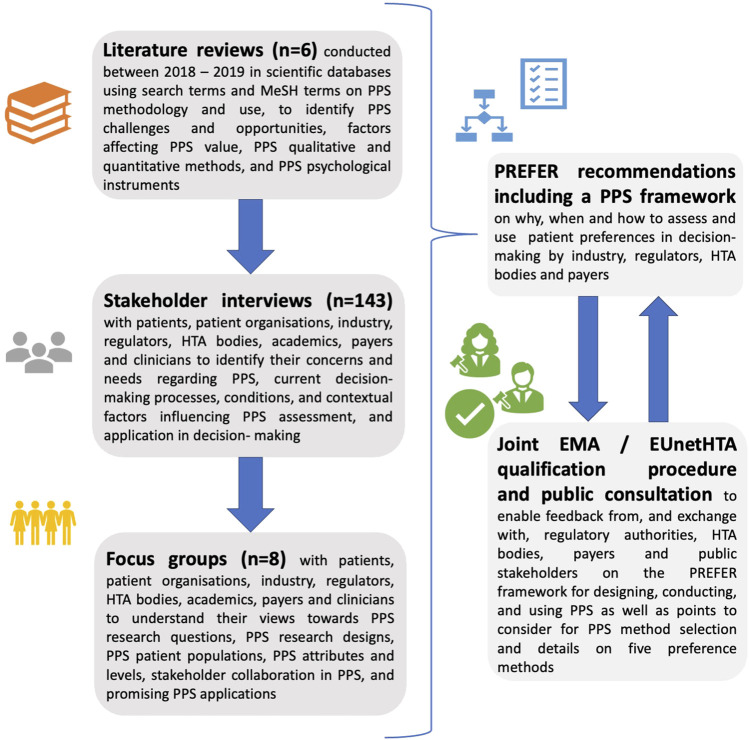
FIGURE 1.
Research design used by PREFER to develop recommendations on how to assess and use patient preferences in medical product decision-making, including in the regulatory evaluation of medicinal products. Six (systematic/scoping) literature reviews, 143 individual stakeholder interviews and eight focus group discussions were held to inform the PREFER recommendations, including a framework for designing, conducting, and using patient preference studies as well as points to consider for method selection and details on five preference methods. Alongside the PREFER recommendations, PREFER undertook a parallel EMA/EUnetHTA Qualification Opinion procedure, which allowed PREFER to exchange with regulatory authorities, HTA bodies, and payers on the recommendations and enabled their input to be incorporated into the recommendations. As part of the methods qualification procedure, the EMA undertook a public consultation to obtain input on the PPS recommendations and framework. PPS, patient preferences studies; EMA, European Medicines Agency; EUnetHTA, European Network for Health Technology Assessment; n, number; HTA, Health Technology Assessment.