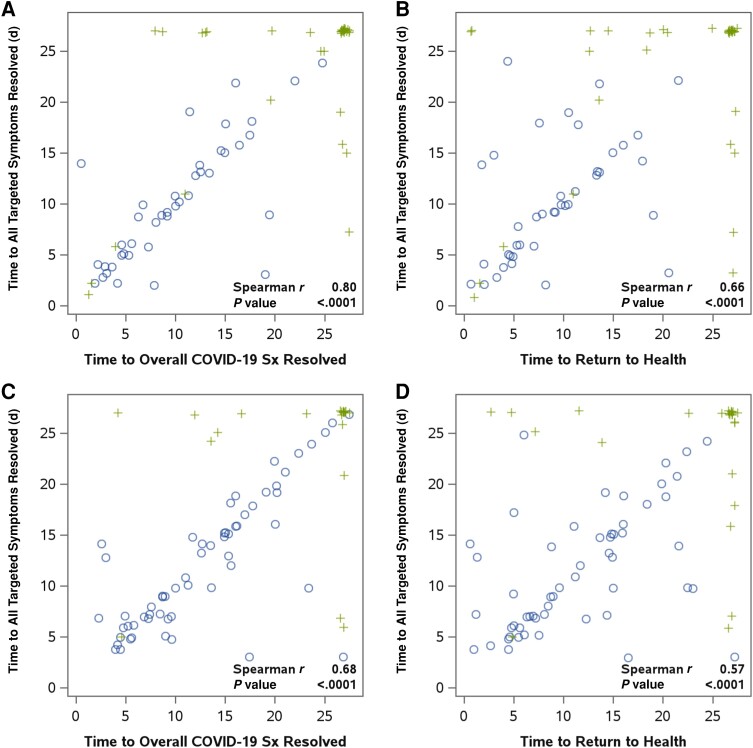
Figure 3.
Internal validity of time to sustained resolution of all targeted symptoms (for 2 days) measure for high- and standard-risk participants. Presented are scatterplots of time to sustained resolution of all targeted symptoms for 2 days (primary outcome measure) against time to sustained resolution of overall COVID-19 symptoms (for 2 days) and time to sustained return to health (for 2 days) for high-risk participants (A and B) and standard-risk participants (C and D). Spearman rank correlations assessed the association between the measures. Circles indicate times that were observed for both outcomes; crosses indicate times that were censored because of end of diary follow-up (values of 27 for 1 or both outcomes) or premature discontinuation of diary completion (values less than 27 for 1 or both outcomes).