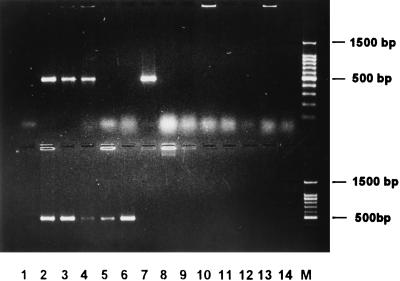
FIG. 2.
P. multocida-specific PCR assay. This figure illustrates fragments specifically amplified by PCR in all P. multocida subspecies and serotypes by means of the primers KMT1SP6 and KMT1T7. The upper panel shows the following: lane 1, negative control; lane 2, P. multocida subsp. multocida; lane 3, P. multocida subsp. gallicida; lane 4, P. multocida subsp. septica; lane 5, Pasteurella dagmatis; lane 6, P. canis biotype 1; lane 7, P. canis biotype 2; lane 8, Pasteurella stomatis; lane 9, Pasteurella anatis; lane 10, Pasteurella langaa; lane 11, Pasteurella species B; lane 12, Pasteurella haemolytica A5; lane 13, Pasteurella haemolytica T10; lane 14, Actinobacillus species 0134; and lane 15, 100-bp DNA marker (Promega). The lower panel shows the following: lane 1, negative control; lane 2, P. multocida Carter type A; lane 3, type B; lane 4, type D; lane 5, type E; lane 6, type F; lane 7, H. influenzae type b; lane 8, A. pleuropneumoniae; lane 9, E. coli; lane 10, Pseudomonas aeruginosa; lane 11, Salmonella typhimurium; lane 12, Staphylococcus aureus; lane 13, Streptococcus faecalis; lane 14, Bacillus cereus; and lane M, 100-bp DNA marker. Samples were electrophoresed at 2 V/cm for 2 h in a 2% agarose gel (1× TAE), stained with ethidium bromide, visualized by UV illumination, and photographed.