Summary
Idiopathic pulmonary fibrosis (IPF) is a highly heterogeneous, unpredictable and ultimately lethal chronic lung disease. Over the last decade, two anti-fibrotic agents have been shown to slow disease progression, however, both drugs are administered uniformly with minimal consideration of disease severity and inter-individual molecular, genetic, and genomic differences. Advances in biological understanding of disease endotyping and the emergence of precision medicine have shown that “a one-size-fits-all approach” to the management of chronic lung diseases is no longer appropriate. While precision medicine approaches have revolutionized the management of other diseases such as lung cancer and asthma, the implementation of precision medicine in IPF clinical practice remains an unmet need despite several reports demonstrating a large number of diagnostic, prognostic and theragnostic biomarker candidates in IPF. This review article aims to summarize our current knowledge of precision medicine in IPF and highlight barriers to translate these research findings into clinical practice.
Keywords: Idiopathic pulmonary fibrosis, Precision medicine, Theragnostic biomarkers, Personalized medicine
Idiopathic pulmonary fibrosis (IPF) is a chronic, debilitating lung disease with increasing prevalence, characterized by a complex interplay of genetic, epigenetic, immunologic and environmental factors.1,2 The disease course is highly heterogeneous and unpredictable. Three distinct patterns of disease progression have been suggested including slowly progressive, rapidly progressive and relatively stable disease interposed by acute exacerbations.3, 4, 5 The last years have seen the emergence of use of two anti-fibrotic agents able to slow disease progression,6,7 however, both drugs are administered uniformly with minimal consideration of differences in molecular subphenotypes associated with disease progression.8,9
Research advances in our understanding of pulmonary fibrosis pathogenesis and progression and the emergence of precision medicine have shown that “a one-size-fits-all approach” to the management of this group of diseases is probably not appropriate.8 Tailored therapies based on precision medicine can improve treatment outcomes and concomitantly be cost-effective through the avoidance of unnecessary exposure of patients to ineffective treatment regimens. In addition to treatment response prediction, precision medicine could also have a major role in the identification of individuals with disease susceptibility and pave the way for early diagnosis. Importantly, precision medicine can discriminate individuals at risk for progressive disease even before progression occurs, providing a window for early intervention or alternative treatment regimens.
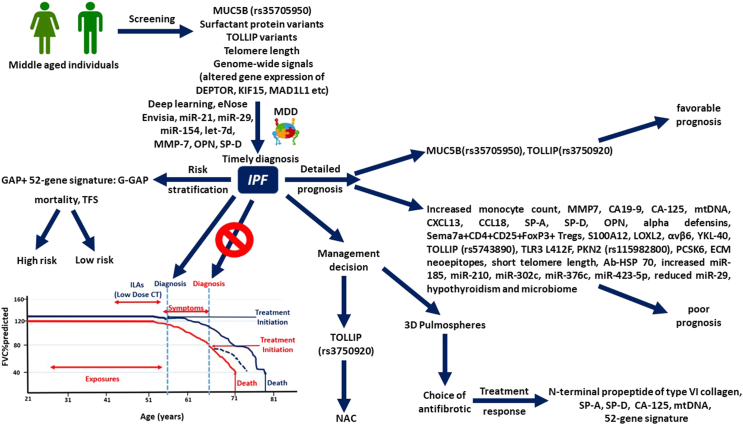
Precision medicine approaches have revolutionized management of lung cancer due to the fact that prognosis and treatment in lung cancer is largely based on patients’ molecular profile.10,11 Recently, a plethora of novel biologic therapies have been approved for severe asthma, such as anti-IL-5/anti-IL-5R and anti-IL4 for patients with eosinophilic predominant severe asthma and anti-IgE for allergic predominant severe asthma with increased IgE.12 Implementation of precision medicine in clinical practice for patients with IPF remains an unmet need. This review article aims to summarize current knowledge for precision medicine in IPF and highlight barriers to overcome for the implementation of these findings in clinical practice (Fig. 1).
Fig. 1.
Schematic representation of personalized medicine approaches that could be implemented in future clinical practice for patients with IPF. Early screening could lead to timely diagnosis, alter the nature course of the disease (red demarcation) and improve outcomes (blue demarcation). Implementation of the 52-gene signature could considerably improve the prognostic performance of GAP index. A plethora of other biomarkers could have prognostic and/or theragnostic role.
Disease susceptibility
Recent studies implicate that IPF is a highly polygenic disease with multiple variants associated with disease susceptibility.13,14 The variant showing the strongest association with pulmonary fibrosis development and pathogenesis is a polymorphism in the promoter region of MUC5B (rs35705950), found using a genome-wide linkage scan in a large-scale study.15, 16, 17, 18, 19 This variant leads to higher MUC5B expression, deregulated mucosal host defense and ultimately increased risk of IPF development almost by 6-fold.16,17,20 Patients with the MUC5B risk allele are less likely to present with telomere-gene mutations.21 Despite the fact that the variant rs35705950 is more common in IPF [38% vs 9% in the general population16], it is present in less than half of the cases. Recent data implicated that MUC5AC may have a role in IPF susceptibility as well, further corroborating the role of mucins in IPF.22 Variants in toll-interacting protein (TOLLIP) leading to reduced expression and a variant of SPPL2C have been also associated with IPF susceptibility in a three-stage Genome-wide association study (GWAS) study.23 On the contrary, another variant of TOLLIP, rs5743890, seemed to be protective against the development of pulmonary fibrosis.23,24
Moreover, large GWAS have demonstrated 20 frequent single nucleotide polymorphisms (SNPs) related to IPF with minor allele frequency above 5%, highlighting the association between disease susceptibility with impaired host defense, cell-to-cell adhesion, signaling and telomere maintenance.13,14,18,23,25,26 In particular, a recent study validated genome-wide significant associations with disease susceptibility for 11 out of the 17 previously published SNPs (7q22.1, AKAP13, ATP11A, DPP9, DSP, FAM13A, IVD, KANSL1, MUC5B, TERC and TERT).18 The same study identified and replicated three novel genome-wide significant SNPs (with associated altered gene expression of DEPTOR, KIF15 and MAD1L1) related to IPF susceptibility.18 DEPTOR inhibits mTOR kinase activity being part of mTORC1 and mTORC2 protein complexes, while TGFb-induced DEPTOR suppression stimulated collagen synthesis.18 Thus, association of decreased DEPTOR expression with increased IPF susceptibility corroborates evidence supporting the cardinal role of mammalian target of rapamycin (mTOR) signaling in pulmonary fibrosis.27 With regards to MAD1L1, it is noteworthy that its homolog MAD1 inhibits TERT activity, which implies that MAD1L1 might increase disease susceptibility via reduced telomerase activity.28,29 Moreover, novel signals of KIF15, SPDL1 and MAD1L1 derived from this work and/or other elegant studies might imply a potentially important role of mitotic spindle-assembly genes in disease susceptibility.18,30,31 An updated meta-analysis of the aforementioned work including five studies demonstrated five robust novel signals (an intergenic variant in 10q25.1, variants in introns of RTEL1, STMN3, KNL1 and NPRL3) further implicating telomere maintenance, mTOR signaling and spindle assembly genes in IPF susceptibility.13 Finally, a large recent meta-analysis including patients from 6 ancestries identified 7 novel IPF loci (index variant gene: GPR157, DNAJB4-GIPC2, RAPGEF2, FKBP5, RP11-286H14.4, PSKH1, FUT6) with 4 of them being driven by non-European ancestry, highlighting thus the differences in terms of genetic background across the world.14
Polymorphisms in several other genes such as transforming growth factor beta-1 (TGFB1), HLA DRB1∗1501, interleukin-1 receptor alpha (IL1RN) and IL8 have been suggested as candidate genes for IPF susceptibility; yet, further larger studies are needed to determine their exact role in disease susceptibility.32, 33, 34, 35, 36 Finally, except variants, polymorphisms and GWAS signals, telomere length has a major role in disease susceptibility. Short telomere length is a frequent finding in patients with IPF compared to aged-matched healthy individuals,37 while interstitial lung abnormalities (ILAs) have been recently associated with decreased mean telomere length.38
Genetic mutations in familial pulmonary fibrosis
While variants (altered genome that could contain one or more mutations and presents with distinct characteristics) have been implicated in sporadic IPF, some mutations (single change in genome that could lead or not to distinct characteristics) have been implicated in familial pulmonary fibrosis. Telomerase complex mutations are more common in familial forms of pulmonary fibrosis and might not be necessarily specific to individual ILD entities.37,39, 40, 41 Accordingly, surfactant protein (SP) mutations including SP-A1, SP-A2 and SP-C42, 43, 44 have been linked to development of Familial Pulmonary Fibrosis, whereas these variants are rarely encountered in sporadic IPF.45, 46, 47 Genome-wide analysis of six families from Finland with Familial Pulmonary Fibrosis suggested ELMOD2, a gene associated with cell migration and phagocytosis of apoptotic cells, as a candidate gene for IPF susceptibility. This gene was expressed in alveolar epithelial cells II and alveolar macrophages. Mutations in ELMOD2 led to reduced ELMOD2 mRNA expression in the pulmonary parenchyma of patients with IPF compared to healthy individuals.48
Taken together, testing for disease susceptibility genes could help personalize the radiological follow-up of individuals at risk for IPF and the treatment of patients with IPF or ILAs. For example, if patients with ILAs have SNPs in the common genes associated with IPF risk, these patients should have more frequent radiologic follow-up compared to patients with ILAs and no genetic variations in IPF susceptibility genes. Further investigation toward this direction is needed.
Diagnosis
Establishing an accurate diagnosis in patients with interstitial lung diseases (ILDs) is often challenging despite the improved quality of High-Resolution Computed Tomography, the advent of deep learning and the multidisciplinary discussion of clinical, laboratory and radiographic data.49, 50, 51 An ideal diagnostic biomarker could reduce rates of misdiagnosis following multidisciplinary discussion and concomitantly provide mechanistic insights for the origin of the disease. The potential of several molecular biomarkers has been investigated in an effort to discriminate patients with IPF from healthy individuals or patients with other ILDs.47,52 Despite an exponential increase in our knowledge regarding IPF pathogenesis, the lack of diagnostic accuracy, disease specificity, applicability and cost-effectiveness of individual biomarkers has been insufficient to justify their incorporation into clinical practice, especially in a setting with limited resources.51 In addition, given the recent guidelines for IPF and Progressive Pulmonary Fibrosis (PPF) and the trend towards lumping rather than splitting, diagnostic biomarkers might be the least pressing need with regards to IPF. However, ruling out other fibrotic ILDs in need of immunosuppressive therapy is still of paramount importance.
A study investigating a panel of 35 extracellular matrix (ECM), ECM-related and lung-specific analytes in plasma showed that matrix metalloproteinase (MMP)-7 > 1.75 ng/ml, SP-D >31 ng/ml and osteopontin >6 ng/ml were able to discriminate patients with IPF from patients with alternative idiopathic interstitial pneumonias, both if used individually and as a combined index.53 However, the same index could not distinguish patients with IPF from patients with rheumatoid arthritis-ILD.53 Data analysis from the IPF-PRO registry showed that patients with IPF have significantly different levels in 551 proteins compared to controls.54 The glycoproteins thrombospondin 1, von Willebrand factor, as well as C–C motif chemokine ligand 17 and bactericidal permeability-increasing protein were among the proteins with the more pronounced difference between IPF and controls54 suggesting coagulation as an important mechanism required for IPF pathogenesis. A targeted proteomic approach in a study with acceptable sample size resulted in a protein signature that included IGFBP-1, MMP-1, MMP-7, MMP-8 and TNFRSA1F and was able to discriminate patients with IPF from control subjects with a sensitivity of 98.6% and a specificity of 98.1%.55 Such approaches with combined indexes increase considerably the diagnostic accuracy of all the above biomarkers compared to results yielded when these biomarkers were investigated individually.53,56, 57, 58, 59
Other biomarkers related to epithelial cells, innate immunity and aging have been investigated mainly on individual basis and not as part of multidimensional indexes. MicroRNAs have also been largely studied as biomarkers for IPF diagnosis. Circulating caspase-cleaved cytokeratin-18, an alveolar epithelial cell apoptosis biomarker, was increased in IPF compared to control subjects.60 The epithelium derived glycoprotein Krebs von den Lungen-6 (KL6)/mucin 1 (MUC1) has been reportedly increased in serum and BAL of patients with IPF47,61,62; yet, high quality studies adjusting for age, smoking and comparing patients with IPF and non-IPF ILDs are still needed. Other studies focusing on the diagnostic potential of epithelium derived proteins demonstrated that SP-A and C-pro-SP-B serum levels were increased in patients with IPF compared to non-IPF ILDs and other pulmonary diseases, respectively.63,64 Investigations of immune deregulation in IPF led to the report that BAL levels of toll-like receptor 7 were higher in IPF compared to controls.24,65 A recent study focusing on biomarkers of aging reported that increased plasma concentration of growth differentiation factor 15 (GDF15), IL-6, tumor necrosis factor α receptor II and C-reactive protein was linked to presence of ILAs.66 Results for GDF15 were validated in a different cohort.66 With regards to miRNAs, miR-29 and let-7d were among the most downregulated, while miR-21 and miR-154 were upregulated in patients with IPF compared to controls.67, 68, 69, 70, 71 Finally, the diagnostic potential of several other biomarkers including secreted phosphoprotein 1 (SPP1), FK506-binding protein 11 and chitinase-3-like protein 1 (YKL-40) has been investigated.72, 73, 74, 75 None of the biomarkers described in this paragraph are currently used in clinical practice but they may have potential for such use with further refinement and development.
To this end, the potential for clinical applicability of most of the aforementioned biomarkers is limited, especially if used individually. Thus, the last years have seen an extensive research effort to investigate the IPF lung tissue, where the disease is identified. Transcriptomic analysis of lung tissue from patients with IPF and Hypersensitivity Pneumonitis showed that genes associated with epithelial development and collagen catabolism were upregulated in both diseases, while immune-response related genes were specifically upregulated in patients with Hypersensitivity Pneumonitis.76 Genomic analysis of lung tissue from transbronchial biopsies resulted in a commercially available biomarker, denominated Envisia Genomic Classifier, with sustained accuracy and high reproducibility for the detection of histopathologic features of usual interstitial pneumonia (UIP).77, 78, 79 Envisia Genomic Classifier might be helpful as a surrogate of histopathology that could improve the diagnostic accuracy in IPF without the need of surgical lung biopsy, if used in a multidisciplinary setting.79 Of course, a major limitation is that identification of UIP through the Envisia Genomic classifier does not necessarily mean IPF. Association of genomic UIP with PPF might substantially increase the clinical applicability of this biomarker: yet, to this end, genomic UIP has not been associated with progression free survival or longitudinal FVC decline.80
Disease severity, risk stratification & outcome prediction
Estimation of disease severity and risk stratification in IPF is still based almost exclusively on functional and physiological indices such as Forced vital capacity (FVC), diffusion capacity for carbon monoxide (DLCO) and 6-min walking test (6MWT) given that computed tomographic biomarkers (deep learning algorithms, e-Lung) are in their infancy.81, 82, 83, 84, 85, 86, 87 Composite physiologic index (CPI) and GAP (Gender, Age and Physiology) index represent two of the most widely used indexes for risk-stratification.88,89 Significant caveats of these indices including the effect of emphysema on FVC, technical variabilities affecting DLCO and the impact of heart-related, myoskeletal disorders on 6MWD highlight the pressing need for disease specific biomarkers.83,90, 91, 92
Gene variations, microRNAs and telomere shortening have all been shown to predict IPF mortality (Table 1). With regards to gene variations, the same MUC5B polymorphism (rs35705950) that led to IPF susceptibility, was paradoxically associated with decreased mortality in IPF.15,131 However, a recent report showed that this finding might be a source of index event bias, a phenomenon observed if subjects are selected based on disease status without accounting for other common causes of incidence and prognosis.132 In terms of other gene variations, the presence of a functional variant of TOLLIP, rs5743890, was associated with reduced survival in IPF.23,133 Similarly, the identification of a TLR3 functional variant (Leu412Phe, TLR3 L412F) in patients with IPF has also been suggested as a marker of progressive disease.125 A recent staged genome-wide association study identified a novel variant, named PCSK6, that reached genome-wide significance.118 PCSK6 which encodes a calcium-dependent serine endoprotease and is mainly expressed in airway epithelial cells, lymphatic endothelial cells and adventitial fibroblasts was associated with increased mortality.118 Another recently identified variant found in an antisense RNA gene of the Rho and Rac effector protein, named protein kinase N2, PKN2, (rs115982800) demonstrated genome-wide significant association with rapid FVC decline119 in IPF. In addition to gene variations, five microRNAs (miR-185, miR-210, miR-302c, miR-376c and miR-423-5p) were increased in IPF lung tissue of rapid compared to slow progressors.111 In terms of peripheral blood, reduced miR-29 expression in serum and plasma was associated with increased mortality in two cohorts of patients with IPF.110 Regarding telomere shortening, shorter telomere length was associated with increased risk of mortality in patients with IPF in independent patient cohorts.122 Additionally, mutations in genes related to telomere maintenance (TERT, TERC, PARN and RTEL1) can be indicative of the PPF phenotype and reduced survival.123
Table 1.
Most important biomarkers studied in IPF and their potential clinical utility.
| Biomarker | Disease susceptibility | Diagnosis | Prognosis | Treatment response/theragnostic | References |
|---|---|---|---|---|---|
| Alpha defensins | + | 56 | |||
| CA 19-9 | + | 59 | |||
| CA-125 | + | + | 59 | ||
| CCL18 | + | 93 | |||
| cCK18 | + | 60 | |||
| CXCL13 | + | 94 | |||
| ECM neoepitopes | + | 95 | |||
| eNose | + | + | 96, 97, 98 | ||
| Envisia Genomic Classifier | + | 77, 78, 79 | |||
| Genome-wide signals (altered gene expression of DEPTOR, KIF15, MAD1L1 etc) | + | 18 | |||
| KL-6/MUC1 | + | + | + | 47,99, 100, 101, 102, 103, 104, 105 | |
| LOXL2 | + | 106 | |||
| MMP7 | + | + | 53,59,107,108 | ||
| Microbiome | + | 109 | |||
| miR-21, miR-154, let-7d | + | 67, 68, 69, 70, 71 | |||
| miR-29 | + | + | 110 | ||
| miR-185, miR-210, miR-302c, miR-376c and miR-423-5p | + | 111 | |||
| Mitochondrial DNA | + | + | 112 | ||
| Monocyte count | + | 113, 114, 115 | |||
| MUC5B | + | + | 15, 16, 17, 18, 19 | ||
| N-terminal propeptide of type VI collagen | + | 116 | |||
| Osteopontin | + | + | 53,117 | ||
| PCSK6 | + | + | + | 118 | |
| PKN2 | + | 119 | |||
| S100A12 | + | 120,121 | |||
| Surfactant proteins | + | + | + | + | 45, 46, 47,53,59 |
| Telomere length/telomerase | + | + | 37,39,122,123 | ||
| Thyroid hormone | + | 124 | |||
| TLR3 | + | 125 | |||
| TOLLIP | + | + | 23,24,126 | ||
| Tregs | + | 127,128 | |||
| 3D pulmospheres | + | 129 | |||
| 52-gene signature | + | + | 9,130 |
Abbreviations: cCK18, caspase-cleaved; CCL18, chemokine ligand 18; CXCL13, CXC-motif ligand 13; ECM, extracellular matrix; KL-6, Krebs von den Lungen-6; LOXL2, lysyl oxidase like-protein-2; MUC, mucin; PKN2, protein kinase 2; SP, surfactant protein; TLR, toll-like receptor; TOLLIP, toll-interacting protein.
In addition to genetics and epigenetic variations, changes in gene expression, particularly in peripheral blood, have also been shown to predict IPF mortality. In particular, one of the studies that provided a significant advance in precision medicine in ILD was the identification of a 52-gene signature in peripheral blood able to predict mortality in IPF in six independent cohorts.9,130 A genomic risk scoring system denominated Scoring Algorithm for Molecular Subphenotypes (SAMS) based on this 52-gene signature was able to discriminate IPF patients into high and low-risk mortality subgroups after adjusting for clinical covariates. The combination of the 52-gene signature risk profiles and GAP index, improved substantially the prognostic accuracy of the GAP index.9 Interestingly, this signature also predicted mortality in COVID-19 suggesting the presence of a profibrotic subphenotype of COVID-19 patients with severe disease.134 Cellular deconvolution of gene expression data not only demonstrated that monocytes are the cellular source of the high-risk profile based on the 52-gene signature but also contributed to the identification of a high monocyte count as predictor of mortality in IPF and other fibrotic disorders.113,134 Large-scale studies have shown that increased monocyte count was linked to increased risk of disease progression, hospitalization, and mortality in IPF.113, 114, 115
Besides monocytes, other peripheral blood and BAL immune cells and biomarkers have been associated with IPF progression and mortality. For example, higher serum levels of chemokine (C-X-C motif) ligand 13 and chemokine ligand 18 (CCL18) were shown to predict disease progression in IPF.93,130,135 Lower CD4 T cell counts and low expression of T-cell co-stimulatory genes are associated with decreased IPF survival.9,130 In terms of regulatory T cells, while BAL Treg proliferative response and IL-4 release were negatively correlated with lung function, Semaphorin 7a+CD4+CD25+FoxP3+ Tregs were increased in the circulation of patients with progressive disease127,128 suggesting immune dysregulation as a mechanism associated with IPF progression. Altered microbiome has been proposed as responsible for immune dysregulation in multiple diseases and could be responsible for the changes seen in IPF.136 For example, increased bacterial burden and specific pathogens in BAL of patients with IPF are predictive of functional decline and death in IPF.109 This could also explain increased expression of the cationic antimicrobial peptides alpha defensins that are mainly expressed in alveolar type II cells and form an essential element of innate immunity, which have been suggested as a marker of acute exacerbation in patients with IPF.56 This finding further highlighted that factors associated with immune deregulation and alveolar epithelial cell injury can be both relevant to IPF pathogenesis and provide prognostic information.
Several studies have looked at the identification of peripheral blood biomarkers reflective of alveolar epithelial cell injury. The PROFILE study, a large prospective study of treatment-naive patients with IPF, focused on epithelium-derived proteins and identified four serum biomarkers (CA19-9, CA-125, MMP-7, SP-D) that had considerable prognostic potential and were suitable for replication.59 Baseline values of SP-D and CA19-9 were higher in patients with the progressive phenotype compared to patients with stability. Increased concentrations of CA-125 over three months were predictive of mortality.59 Increased concentrations of MMP7 were associated with worse survival.59 The aforementioned results were in line with other studies showing the negative prognostic of higher SP-D, SP-A, MMP-7 and other metalloproteinases in IPF.59,137, 138, 139, 140 Higher levels of osteopontin, another protein mainly expressed in alveolar epithelial cells, might be a marker of acute exacerbations in patients with IPF.56,117 ELISA obtained values of osteopontin, MMP-7, periostin and ICAM1 led also to a progression index able to discriminate patients with stable and progressive disease.141 Increased KL-6, which is also a protein reflecting injury of alveolar epithelial cells type II, has been suggested as a marker of disease progression and mortality47,99; yet, results were not reproducible in other studies.47,100,142 Serial measurements of KL-6 might be the key for the optimization of the prognostic value of this biomarker.101,102 Most recently, increased KL-6 was demonstrated as a prognosticator of acute exacerbations.100,103,104
Investigations related with the metabolic state of alveolar epithelial cells yielded also important findings. The evidence that hypothyroidism predicted mortality in patients with IPF coupled with experimental data showing that thyroid hormone improved epithelial mitochondrial function.124,143 Further research effort to obtain prognostic information through studying metabolic derangements showed that increased mitochondrial DNA correlated with poor survival in two cohorts of patients with IPF.112 Studies focusing on biomarkers relevant to collagen demonstrated also interesting results. Another report analyzing data from the PROFILE study investigated longitudinal change in collagen degradation biomarkers and showed that extracellular matrix neoepitopes were associated with disease progression.95 Higher serum levels of lysyl oxidase-like 2, a protein promoting collagen accumulation, have been associated with IPF progression106; yet, findings require validation. Several other non-disease specific biomarkers, including periostin, YKL-40, S100A12, ανβ6 integrin and anti-heat shock protein 70 have been suggested as prognostic markers in IPF3,47,52,62,120,121,133,144, 145, 146, 147, 148 (Table 1). Further studies focusing on the implementation of these prognostic biomarkers are required to monitor disease progression, timing for lung transplant referrals and treatment decisions.
Prediction of treatment response—theragnostic biomarkers
There is still a pressing need of biomarkers able to predict and measure treatment response in IPF (Table 2). One of the first studies demonstrating the applicability of theragnostic biomarkers in IPF showed that TOLLIP CC genotype and TT genotype were associated with worst and better N-Acetylcysteine (NAC) response, respectively.126 The main limitations of this study are 1) that genetic data were available for a small number of patients in this study and 2) that NAC is not currently FDA approved to be used as a treatment for patients with IPF. Results from the PRECISIONS trial which seeks to address whether NAC has a differential effect on lung fibrosis progression depending on TOLLIP gene variants are greatly anticipated.154 Currently, only two drugs, nintedanib and pirfenidone are FDA approved to slow lung function decline in IPF.
Table 2.
Biomarkers able to predict or measure treatment response in pulmonary fibrosis.
| Biomarker | Compounds | Exact role | References |
|---|---|---|---|
| CA-125 | Nintedanib | CA-125 may be a biomarker of response to nintedanib Adjusted mean CA-125 levels decreased with nintedanib vs placebo from week 4 |
59,149 |
| ECM turnover biomarkers | Nintedanib | Potential as theragnostic biomarkers | 150 |
| eNose | Both antifibrotics | eNose technology may predict treatment response | 96 |
| KL-6/MUC1 | Pirfenidone | Levels might correlate with pirfenidone response; further studies are needed | 105 |
| Mitochondrial DNA | Pirfenidone | Change following 3 months of treatment correlated with pirfenidone response after 1 year | 112 |
| N-terminal propeptide of type VI collagen | Nintedanib | Nintedanib reduced levels as early as week 4 | 116 |
| SP-A | Both antifibrotics | Decrease baseline to 3 months and 6 months predicted outcomes at 6 months; larger studies are needed | 151 |
| SP-D | Both antifibrotics | Nintedanib reduced levels as early as week 4; may be a pharmacodynamic biomarker of pirfenidone | 116,152,153 |
| TOLLIP TT genotype | NAC | Better response in NAC | 126 |
| 3D pulmospheres | Both antifibrotics | Invasiveness predicted antifibrotic responsiveness | 129 |
| 52-gene signature | Both antifibrotics | Genomic risk profiles shifted their trends over time following antifibrotic treatment | 9,130 |
Abbreviations: cCK18, caspase-cleaved; CCL18, chemokine ligand 18; CXCL13, CXC-motif ligand 13; ECM, extracellular matrix; KL-6, Krebs von den Lungen-6; LOXL2, lysyl oxidase like-protein-2; MUC, mucin; PKN2, protein kinase 2; SP, surfactant protein; TLR, toll-like receptor; TOLLIP, toll-interacting protein.
In terms of theragnostic biomarkers able to measure treatment response to nintedanib and pirfenidone in IPF, the N-terminal propeptide of type VI collagen and SP-D may be adequate theragnostic biomarkers given that nintedanib significantly reduced levels of both biomarkers as early as week 4.116 CA-125 holds potential as a marker of response to nintedanib and further data are greatly anticipated.59 Serum SP-D might be a biomarker for pirfenidone effectiveness as well152 as a potential pharmacodynamic biomarker, especially when measured serially.153 Change in mitochondrial DNA following 3 months of treatment correlated with pirfenidone response after 1 year.112 Finally, KL-6 and CCL18 were able to predict disease progression and mortality in IPF; yet, more studies are needed to address if KL-6 can be used as a theragnostic biomarker, while CCL-18 failed to predict treatment response.105,147,155,156
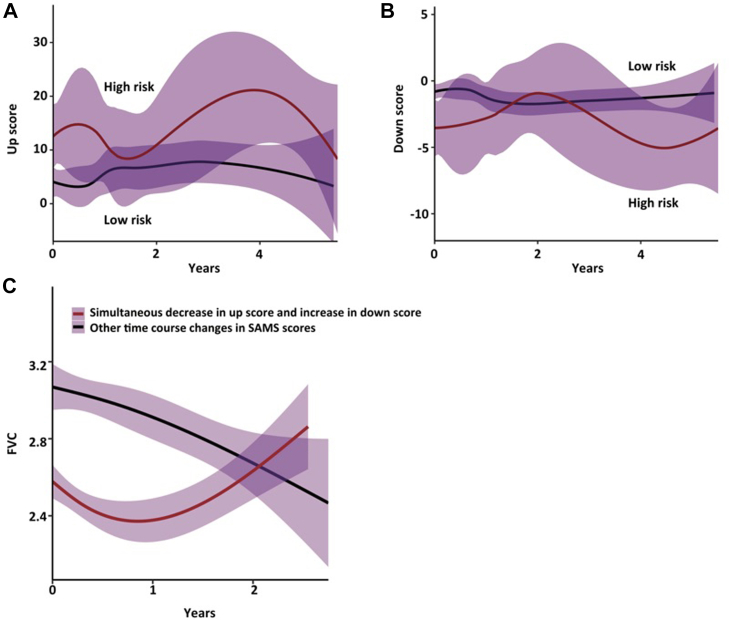
Other studies have investigated the theragnostic potential of certain biomarkers in IPF. For example, a study limited by sample size suggested that SP-A might have theragnostic potential both for nintedanib and pirfenidone.151 Analysis of the INMARK trial with regards to the change of biomarkers following treatment with nintedanib, showed that ECM turnover biomarkers such as collagen 1 degraded by MMP (C1M), collagen 6 degraded by MMP-2/9 (C6M), collagen 5 degraded by MMP-2/9 (C5M) and C reactive protein degraded by MMP-1/8 (CRPM) might be theragnostic biomarkers; yet, further investigation is needed.150 Importantly, a novel study suggested a novel way to simulate lung microenvironment through 3D pulmospheres, which are spheroids consisting of cells from primary lung biopsy. Quantification of 3D pulmospheres invasiveness, defined as the zone of invasion percentage, was a reliable way to assess responsiveness in antifibrotics and thus this approach holds promise for the guidance of treatment decision toward the antifibrotic that is more likely to confer benefit to each individual. A major caveat hampering the clinical applicability of that work is that pulmospheres were obtained via video-assisted thoracic surgery.129 Forming 3D pulmospheres with tissue derived from less invasive methods such as cryobiopsy might be the key to implement such personalized medicine approaches in the future clinical practice. Recent data showed that eNose technology may predict treatment response to antifibrotics before treatment initiation.96, 97, 98 A clinical molecular signature of CA-125, CXCL13, MMP-7, OPN and YKL-40 that predicted differential transplant free survival in untreated patients with IPF, was able to retain its prognostic accuracy (but at higher thresholds) in patients receiving antifibrotics.94 Finally, the 52-gene signature that was predictive of mortality in the peripheral blood in IPF, showed also potential as a biomarker of treatment response, given that genomic risk profiles shifted their trends over time after initiation of antifibrotic therapy (Fig. 2).
Fig. 2.
52-gene signature trends in high-risk patients with IPF shift after anti-fibrotic therapy initiation. Panel A and B show up and down scores derived from SAMS respectively. Scores shift their trends over time in high (continuous red line) vs low (continuous black line) risk groups after antifibrotic initiation. Panel C shows FVC trends of treated patients. A simultaneous reduction in up score and increase in down score is shown with black line, while other score changes are shown with red lines. (Modified from the article of Herazo-Maya et al, Lancet Respiratory Medicine 2017 with permission.)
Future perspectives and concluding remarks
During the last decade, we have witnessed a scientific explosion leading to several biomarkers and two anti-fibrotic compounds able to slow IPF progression (Tables 1, 2, and 3, Fig. 1). The new challenge is the translation of biomarkers that have acceptable cost and are able to provide actionable information to clinicians. A really important biomarker should alter clinicians’ decision i.e. by leading to early diagnosis, providing information regarding disease activity or the need for treatment modification. This could be achieved through single biomarkers, multidimensional indexes or polygenic risk scores (scores that identify individual's risk based on the combination of different gene abnormalities associated to pulmonary fibrosis) like the one recently presented in the form of an abstract.157
Table 3.
Main mechanistic pathways related to biomarkers in IPF.
| Mechanistic pathway | Biomarkers |
|---|---|
| Alveolar epithelial dysfunction | Alpha defensins, CA19-9, CA-125, CK18, KL-6/MUC1, MUC5B, PCSK6, osteopontin, SP-A, SP-C, SP-D, telomeres, telomerase |
| Immune dysregulation | Alpha defensins, CXCL13, CCL18, HSP70, microbiome, monocyte count, S100A12, TLR3, TOLLIP, Tregs, 52-gene signature |
| Extracellular matrix remodeling | Collagen degradation biomarkers, ECM neoepitopes, LOXL2, MMP7, PCSK6 |
| Epigenetic markers | miR-29, let-7d, miR-21, miR-154, miR-302c, miR-423, miR-210, miR-376c, miR-185 |
| Metabolism | Thyroid hormone, mitochondrial DNA |
Abbreviations: ECM, extracellular matrix; KL-6, Krebs von den Lungen-6; MUC, mucin; SP, surfactant protein; TOLLIP, toll-interacting protein.
Biomarkers that correlate with disease activity could be a major tool to identify the time to intervene. These biomarkers could have a major role for patients ILAs and mild functional impairment, given that in these cases antifibrotic treatment is sometimes delayed. Moreover, biomarkers that can lead to identification of specific endotypes will be important.15,122,158,159 Classifying pulmonary fibrosis as high-risk genomic PF, MUC5B-PF or telomeropathy-PF instead of using the word “idiopathic” might be a better approach. The use of precision medicine and endotyping could pave the way to pharmacogenetic approaches and guide treatment decisions. Treating endotypes with targeted therapies based on the expression of specific biomarkers could maximize effectiveness of future therapies and concomitantly spare adverse events. Current examples of this are 1) the clinical trial testing the synthetic androgen danazol for patients with short telomeres based on previous reports that androgens can restore telomerase activity in IPF and 2) the PRECISIONS trial for NAC based on TOLLIP gene variants.160, 161, 162 Moving from a uniform approach to a patient-centered approach is critical.163,164 Trials of theragnostic biomarkers along with weight-based dosage of antifibrotics or trials of lower doses in patients with ILAs might confer benefit and concomitantly spare the adverse events that lead to treatment discontinuation. Studies aiming to manage symptoms, in a personalized fashion, should be strongly encouraged. For example, the extended-release form of nalbuphine, a dual-acting k opioid receptor agonist/μ opioid receptor antagonist holds promise as a compound able to reduce chronic cough in patients with IPF.165
Taken together, considerable progress has been made in the area of precision medicine in IPF. Nonetheless, there is a need for high-quality, implementation research to bring these biomarkers into daily clinical practice. Of course, only a substantial minority of the aforementioned biomarkers will ultimately be applied in the clinical practice. Probably these biomarkers will be cost-effective and able to provide actionable information. Future clinical trials for new compounds should focus on disease endotypes and pharmacogenetics. They should also include disease severity and theragnostic biomarkers. Such approaches will require significant investment but will lead to improvement in quality of life and better patient outcomes.
Outstanding questions.
-
1.
Should we classify fibrotic ILDs based on their endotype using terms such as MUC5B-PF or telomeropathy-PF instead of using the word “idiopathic”?
-
2.
Could biomarker-based clinical trials lead to the approval of novel compounds for specific subpopulations?
-
3.
Should we treat ILAs with lower doses of antifibrotics in order to slow disease progression and reduce the likelihood of adverse events?
Search strategy and selection criteria.
Data for this Review were identified by searches of MEDLINE, PubMed and references from relevant articles using the search terms “precision medicine in IPF”, “personalized medicine in IPF”, “pathogenesis of pulmonary fibrosis”, “biomarkers in pulmonary fibrosis”, “epithelial cells in pulmonary fibrosis”, “extracellular matrix”, “metabolism in pulmonary fibrosis”, “genetics and epigenetics in pulmonary fibrosis”, “immunity in pulmonary fibrosis”, “diagnostic biomarkers in pulmonary fibrosis”, “prognostic biomarkers in pulmonary fibrosis” and “theragnostic biomarkers in pulmonary fibrosis”. Abstracts and reports from meetings were included only when they related directly to previously published work. Only articles published in English between 2000 and 2023 were included.
Contributors
Theodoros Karampitsakos: conceptualisation, investigation, methodology, visualisation, writing—original draft.
Brenda M. Juan-Guardela: investigation, methodology, visualisation, writing—review & editing.
Argyris Tzouvelekis: conceptualisation, investigation, methodology, visualisation, project administration, supervision, writing—original draft.
Jose D. Herazo-Maya: conceptualisation, investigation, methodology, visualisation, project administration, supervision, writing—original draft.
All authors approved final form and offered significant intellectual contribution.
Declaration of interests
None to declare.
Acknowledgements
This work was supported by the Ubben Family Fund (#250392). The funders did not have any role in paper design, data collection, data analysis, interpretation and writing of the paper.
References
- 1.Maher T.M., Bendstrup E., Dron L., et al. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir Res. 2021;22(1):197. doi: 10.1186/s12931-021-01791-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herazo-Maya J.D., Kaminski N. Personalized medicine: applying 'omics' to lung fibrosis. Biomark Med. 2012;6(4):529–540. doi: 10.2217/bmm.12.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tzouvelekis A., Herazo-Maya J., Sakamoto K., Bouros D. Biomarkers in the evaluation and management of idiopathic pulmonary fibrosis. Curr Top Med Chem. 2016;16(14):1587–1598. doi: 10.2174/1568026616666150930120959. [DOI] [PubMed] [Google Scholar]
- 4.Spagnolo P., Sverzellati N., Rossi G., et al. Idiopathic pulmonary fibrosis: an update. Ann Med. 2015;47(1):15–27. doi: 10.3109/07853890.2014.982165. [DOI] [PubMed] [Google Scholar]
- 5.Rosas I.O., Kaminski N. Update in diffuse parenchymal lung disease 2013. Am J Respir Crit Care Med. 2015;191(3):270–274. doi: 10.1164/rccm.201405-0856UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maher T.M., Lancaster L.H., Jouneau S., et al. Pirfenidone treatment in individuals with idiopathic pulmonary fibrosis: impact of timing of treatment initiation. Ann Am Thorac Soc. 2019;16(7):927–930. doi: 10.1513/AnnalsATS.201810-720RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lancaster L., Crestani B., Hernandez P., et al. Safety and survival data in patients with idiopathic pulmonary fibrosis treated with nintedanib: pooled data from six clinical trials. BMJ Open Respir Res. 2019;6(1) doi: 10.1136/bmjresp-2018-000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maher T.M. Precision medicine in idiopathic pulmonary fibrosis. QJM. 2016;109(9):585–587. doi: 10.1093/qjmed/hcw117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herazo-Maya J.D., Sun J., Molyneaux P.L., et al. Validation of a 52-gene risk profile for outcome prediction in patients with idiopathic pulmonary fibrosis: an international, multicentre, cohort study. Lancet Respir Med. 2017;5(11):857–868. doi: 10.1016/S2213-2600(17)30349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M., Herbst R.S., Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. 2021;27(8):1345–1356. doi: 10.1038/s41591-021-01450-2. [DOI] [PubMed] [Google Scholar]
- 11.Karampitsakos T., Spagnolo P., Mogulkoc N., et al. Lung cancer in patients with idiopathic pulmonary fibrosis: a retrospective multicentre study in Europe. Respirology. 2023;28(1):56–65. doi: 10.1111/resp.14363. [DOI] [PubMed] [Google Scholar]
- 12.Brusselle G.G., Koppelman G.H. Biologic therapies for severe asthma. N Engl J Med. 2022;386(2):157–171. doi: 10.1056/NEJMra2032506. [DOI] [PubMed] [Google Scholar]
- 13.Allen R.J., Stockwell A., Oldham J.M., et al. Genome-wide association study across five cohorts identifies five novel loci associated with idiopathic pulmonary fibrosis. Thorax. 2022;77(8):829–833. doi: 10.1136/thoraxjnl-2021-218577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Partanen J.J., Häppölä P., Zhou W., et al. Leveraging global multi-ancestry meta-analysis in the study of idiopathic pulmonary fibrosis genetics. Cell Genom. 2022;2(10) doi: 10.1016/j.xgen.2022.100181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peljto A.L., Zhang Y., Fingerlin T.E., et al. Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. JAMA. 2013;309(21):2232–2239. doi: 10.1001/jama.2013.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seibold M.A., Wise A.L., Speer M.C., et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364(16):1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunninghake G.M., Hatabu H., Okajima Y., et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med. 2013;368(23):2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen R.J., Guillen-Guio B., Oldham J.M., et al. Genome-wide association study of susceptibility to idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2020;201(5):564–574. doi: 10.1164/rccm.201905-1017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juge P.-A., Lee J.S., Ebstein E., et al. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med. 2018;379(23):2209–2219. doi: 10.1056/NEJMoa1801562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borie R., Cardwell J., Konigsberg I.R., et al. Colocalization of gene expression and DNA methylation with genetic risk variants supports functional roles of MUC5B and DSP in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2022;206(10):1259–1270. doi: 10.1164/rccm.202110-2308OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dressen A., Abbas A.R., Cabanski C., et al. Analysis of protein-altering variants in telomerase genes and their association with MUC5B common variant status in patients with idiopathic pulmonary fibrosis: a candidate gene sequencing study. Lancet Respir Med. 2018;6(8):603–614. doi: 10.1016/S2213-2600(18)30135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorenzo-Salazar J.M., Ma S.F., Jou J., et al. Novel idiopathic pulmonary fibrosis susceptibility variants revealed by deep sequencing. ERJ Open Res. 2019;5(2) doi: 10.1183/23120541.00071-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noth I., Zhang Y., Ma S.F., et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med. 2013;1(4):309–317. doi: 10.1016/S2213-2600(13)70045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karampitsakos T., Woolard T., Bouros D., Tzouvelekis A. Toll-like receptors in the pathogenesis of pulmonary fibrosis. Eur J Pharmacol. 2017;808:35–43. doi: 10.1016/j.ejphar.2016.06.045. [DOI] [PubMed] [Google Scholar]
- 25.Mushiroda T., Wattanapokayakit S., Takahashi A., et al. A genome-wide association study identifies an association of a common variant in TERT with susceptibility to idiopathic pulmonary fibrosis. J Med Genet. 2008;45(10):654–656. doi: 10.1136/jmg.2008.057356. [DOI] [PubMed] [Google Scholar]
- 26.Fingerlin T.E., Murphy E., Zhang W., et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45(6):613–620. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodcock H.V., Eley J.D., Guillotin D., et al. The mTORC1/4E-BP1 axis represents a critical signaling node during fibrogenesis. Nat Commun. 2019;10(1):6. doi: 10.1038/s41467-018-07858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coe B.P., Lee E.H., Chi B., et al. Gain of a region on 7p22.3, containing MAD1L1, is the most frequent event in small-cell lung cancer cell lines. Genes Chromosomes Cancer. 2006;45(1):11–19. doi: 10.1002/gcc.20260. [DOI] [PubMed] [Google Scholar]
- 29.Kang J.U., Koo S.H., Kwon K.C., Park J.W., Kim J.M. Gain at chromosomal region 5p15.33, containing TERT, is the most frequent genetic event in early stages of non-small cell lung cancer. Cancer Genet Cytogenet. 2008;182(1):1–11. doi: 10.1016/j.cancergencyto.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Zhang D., Povysil G., Kobeissy P.H., et al. Rare and common variants in KIF15 contribute to genetic risk of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2022;206(1):56–69. doi: 10.1164/rccm.202110-2439OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhindsa R.S., Mattsson J., Nag A., et al. Identification of a missense variant in SPDL1 associated with idiopathic pulmonary fibrosis. Commun Biol. 2021;4(1):392. doi: 10.1038/s42003-021-01910-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kropski J.A., Blackwell T.S., Loyd J.E. The genetic basis of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(6):1717–1727. doi: 10.1183/09031936.00163814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Son J.Y., Kim S.Y., Cho S.H., et al. TGF-β1 T869C polymorphism may affect susceptibility to idiopathic pulmonary fibrosis and disease severity. Lung. 2013;191(2):199–205. doi: 10.1007/s00408-012-9447-z. [DOI] [PubMed] [Google Scholar]
- 34.Whyte M., Hubbard R., Meliconi R., et al. Increased risk of fibrosing alveolitis associated with interleukin-1 receptor antagonist and tumor necrosis factor-alpha gene polymorphisms. Am J Respir Crit Care Med. 2000;162(2 Pt 1):755–758. doi: 10.1164/ajrccm.162.2.9909053. [DOI] [PubMed] [Google Scholar]
- 35.Ahn M.H., Park B.L., Lee S.H., et al. A promoter SNP rs4073T>A in the common allele of the interleukin 8 gene is associated with the development of idiopathic pulmonary fibrosis via the IL-8 protein enhancing mode. Respir Res. 2011;12(1):73. doi: 10.1186/1465-9921-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue J., Gochuico B.R., Alawad A.S., et al. The HLA class II allele DRB1∗1501 is over-represented in patients with idiopathic pulmonary fibrosis. PLoS One. 2011;6(2) doi: 10.1371/journal.pone.0014715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cronkhite J.T., Xing C., Raghu G., et al. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178(7):729–737. doi: 10.1164/rccm.200804-550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Putman R.K., Axelsson G.T., Ash S.Y., et al. Interstitial lung abnormalities are associated with decreased mean telomere length. Eur Respir J. 2022;60(2) doi: 10.1183/13993003.01814-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armanios M.Y., Chen J.J., Cogan J.D., et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356(13):1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 40.Rode L., Bojesen S.E., Weischer M., Vestbo J., Nordestgaard B.G. Short telomere length, lung function and chronic obstructive pulmonary disease in 46,396 individuals. Thorax. 2013;68(5):429–435. doi: 10.1136/thoraxjnl-2012-202544. [DOI] [PubMed] [Google Scholar]
- 41.Duckworth A., Gibbons M.A., Allen R.J., et al. Telomere length and risk of idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease: a mendelian randomisation study. Lancet Respir Med. 2021;9(3):285–294. doi: 10.1016/S2213-2600(20)30364-7. [DOI] [PubMed] [Google Scholar]
- 42.Nogee L.M., Dunbar A.E., 3rd, Wert S.E., Askin F., Hamvas A., Whitsett J.A. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344(8):573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 43.Liu L., Qin J., Guo T., et al. Identification and functional characterization of a novel surfactant protein A2 mutation (p.N207Y) in a Chinese family with idiopathic pulmonary fibrosis. Mol Genet Genomic Med. 2020;8(9) doi: 10.1002/mgg3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nathan N., Giraud V., Picard C., et al. Germline SFTPA1 mutation in familial idiopathic interstitial pneumonia and lung cancer. Hum Mol Genet. 2016;25(8):1457–1467. doi: 10.1093/hmg/ddw014. [DOI] [PubMed] [Google Scholar]
- 45.Lawson W.E., Grant S.W., Ambrosini V., et al. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax. 2004;59(11):977–980. doi: 10.1136/thx.2004.026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Moorsel C.H., Ten Klooster L., van Oosterhout M.F., et al. SFTPA2 mutations in familial and sporadic idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2015;192(10):1249–1252. doi: 10.1164/rccm.201504-0675LE. [DOI] [PubMed] [Google Scholar]
- 47.Stainer A., Faverio P., Busnelli S., et al. Molecular biomarkers in idiopathic pulmonary fibrosis: state of the art and future directions. Int J Mol Sci. 2021;22(12):6255. doi: 10.3390/ijms22126255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hodgson U., Pulkkinen V., Dixon M., et al. ELMOD2 is a candidate gene for familial idiopathic pulmonary fibrosis. Am J Hum Genet. 2006;79(1):149–154. doi: 10.1086/504639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walsh S.L.F., Calandriello L., Silva M., Sverzellati N. Deep learning for classifying fibrotic lung disease on high-resolution computed tomography: a case-cohort study. Lancet Respir Med. 2018;6(11):837–845. doi: 10.1016/S2213-2600(18)30286-8. [DOI] [PubMed] [Google Scholar]
- 50.Karampitsakos T., Kalogeropoulou C., Tzilas V., et al. Safety and effectiveness of mycophenolate mofetil in interstitial lung diseases: insights from a machine learning radiographic model. Respiration. 2022;101(3):262–271. doi: 10.1159/000519215. [DOI] [PubMed] [Google Scholar]
- 51.Raghu G., Remy-Jardin M., Richeldi L., et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205(9):e18–e47. doi: 10.1164/rccm.202202-0399ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bowman W.S., Echt G.A., Oldham J.M. Biomarkers in progressive fibrosing interstitial lung disease: optimizing diagnosis, prognosis, and treatment response. Front Med. 2021;8 doi: 10.3389/fmed.2021.680997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White E.S., Xia M., Murray S., et al. Plasma surfactant protein-D, matrix metalloproteinase-7, and osteopontin index distinguishes idiopathic pulmonary fibrosis from other idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2016;194(10):1242–1251. doi: 10.1164/rccm.201505-0862OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Todd J.L., Neely M.L., Overton R., et al. Peripheral blood proteomic profiling of idiopathic pulmonary fibrosis biomarkers in the multicentre IPF-PRO registry. Respir Res. 2019;20(1):227. doi: 10.1186/s12931-019-1190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosas I.O., Richards T.J., Konishi K., et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008;5(4) doi: 10.1371/journal.pmed.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Konishi K., Gibson K.F., Lindell K.O., et al. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;180(2):167–175. doi: 10.1164/rccm.200810-1596OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morais A., Beltrão M., Sokhatska O., et al. Serum metalloproteinases 1 and 7 in the diagnosis of idiopathic pulmonary fibrosis and other interstitial pneumonias. Respir Med. 2015;109(8):1063–1068. doi: 10.1016/j.rmed.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Cosgrove G.P., Schwarz M.I., Geraci M.W., Brown K.K., Worthen G.S. Overexpression of matrix metalloproteinase-7 in pulmonary fibrosis. Chest. 2002;121(3 Suppl):25s–26s. [PubMed] [Google Scholar]
- 59.Maher T.M., Oballa E., Simpson J.K., et al. An epithelial biomarker signature for idiopathic pulmonary fibrosis: an analysis from the multicentre PROFILE cohort study. Lancet Respir Med. 2017;5(12):946–955. doi: 10.1016/S2213-2600(17)30430-7. [DOI] [PubMed] [Google Scholar]
- 60.Cha S.I., Ryerson C.J., Lee J.S., et al. Cleaved cytokeratin-18 is a mechanistically informative biomarker in idiopathic pulmonary fibrosis. Respir Res. 2012;13(1):105. doi: 10.1186/1465-9921-13-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohnishi H., Yokoyama A., Kondo K., et al. Comparative study of KL-6, surfactant protein-A, surfactant protein-D, and monocyte chemoattractant protein-1 as serum markers for interstitial lung diseases. Am J Respir Crit Care Med. 2002;165(3):378–381. doi: 10.1164/ajrccm.165.3.2107134. [DOI] [PubMed] [Google Scholar]
- 62.Spagnolo P., Tzouvelekis A., Maher T.M. Personalized medicine in idiopathic pulmonary fibrosis: facts and promises. Curr Opin Pulm Med. 2015;21(5):470–478. doi: 10.1097/MCP.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 63.Wang K., Ju Q., Cao J., Tang W., Zhang J. Impact of serum SP-A and SP-D levels on comparison and prognosis of idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Medicine. 2017;96(23) doi: 10.1097/MD.0000000000007083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kahn N., Rossler A.K., Hornemann K., et al. C-proSP-B: a possible biomarker for pulmonary diseases? Respiration. 2018;96(2):117–126. doi: 10.1159/000488245. [DOI] [PubMed] [Google Scholar]
- 65.Margaritopoulos G.A., Antoniou K.M., Karagiannis K., et al. Investigation of toll-like receptors in the pathogenesis of fibrotic and granulomatous disorders: a bronchoalveolar lavage study. Fibrogenesis Tissue Repair. 2010;3:20. doi: 10.1186/1755-1536-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanders J.L., Putman R.K., Dupuis J., et al. The association of aging biomarkers, interstitial lung abnormalities, and mortality. Am J Respir Crit Care Med. 2021;203(9):1149–1157. doi: 10.1164/rccm.202007-2993OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tzouvelekis A., Kaminski N. Epigenetics in idiopathic pulmonary fibrosis. Biochem Cell Biol. 2015;93(2):159–170. doi: 10.1139/bcb-2014-0126. [DOI] [PubMed] [Google Scholar]
- 68.Pandit K.V., Corcoran D., Yousef H., et al. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;182(2):220–229. doi: 10.1164/rccm.200911-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cushing L., Kuang P.P., Qian J., et al. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45(2):287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu G., Friggeri A., Yang Y., et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207(8):1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Milosevic J., Pandit K., Magister M., et al. Profibrotic role of miR-154 in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;47(6):879–887. doi: 10.1165/rcmb.2011-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liao Y., Wang R., Wen F. Diagnostic and prognostic value of secreted phosphoprotein 1 for idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Biomarkers. 2022;28(1):87–96. doi: 10.1080/1354750X.2022.2148744. [DOI] [PubMed] [Google Scholar]
- 73.Tong X., Ma Y., Liu T., et al. Can YKL-40 be used as a biomarker for interstitial lung disease?: A systematic review and meta-analysis. Medicine. 2021;100(17) doi: 10.1097/MD.0000000000025631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Preisendörfer S., Ishikawa Y., Hennen E., et al. FK506-binding protein 11 is a novel plasma cell-specific antibody folding catalyst with increased expression in idiopathic pulmonary fibrosis. Cells. 2022;11(8):1341. doi: 10.3390/cells11081341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Staab-Weijnitz C.A., Fernandez I.E., Knuppel L., et al. FK506-binding protein 10, a potential novel drug target for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2015;192(4):455–467. doi: 10.1164/rccm.201412-2233OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Furusawa H., Cardwell J.H., Okamoto T., et al. Chronic hypersensitivity pneumonitis, an interstitial lung disease with distinct molecular signatures. Am J Respir Crit Care Med. 2020;202(10):1430–1444. doi: 10.1164/rccm.202001-0134OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pankratz D.G., Choi Y., Imtiaz U., et al. Usual interstitial pneumonia can be detected in transbronchial biopsies using machine learning. Ann Am Thorac Soc. 2017;14(11):1646–1654. doi: 10.1513/AnnalsATS.201612-947OC. [DOI] [PubMed] [Google Scholar]
- 78.Raghu G., Flaherty K.R., Lederer D.J., et al. Use of a molecular classifier to identify usual interstitial pneumonia in conventional transbronchial lung biopsy samples: a prospective validation study. Lancet Respir Med. 2019;7(6):487–496. doi: 10.1016/S2213-2600(19)30059-1. [DOI] [PubMed] [Google Scholar]
- 79.Richeldi L., Scholand M.B., Lynch D.A., et al. Utility of a molecular classifier as a complement to high-resolution computed tomography to identify usual interstitial pneumonia. Am J Respir Crit Care Med. 2021;203(2):211–220. doi: 10.1164/rccm.202003-0877OC. [DOI] [PubMed] [Google Scholar]
- 80.Chaudhary S., Weigt S.S., Ribeiro Neto M.L., et al. Interstitial lung disease progression after genomic usual interstitial pneumonia testing. Eur Respir J. 2023;61(4) doi: 10.1183/13993003.01245-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raghu G., Collard H.R., Egan J.J., et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Richeldi L., Collard H.R., Jones M.G. Idiopathic pulmonary fibrosis. Lancet. 2017;389(10082):1941–1952. doi: 10.1016/S0140-6736(17)30866-8. [DOI] [PubMed] [Google Scholar]
- 83.du Bois R.M., Albera C., Bradford W.Z., et al. 6-minute walk test distance is an independent predictor of mortality in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2013;43(5):1421–1429. doi: 10.1183/09031936.00131813. [DOI] [PubMed] [Google Scholar]
- 84.du Bois R.M., Weycker D., Albera C., et al. Six-minute-walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med. 2011;183(9):1231–1237. doi: 10.1164/rccm.201007-1179OC. [DOI] [PubMed] [Google Scholar]
- 85.Nathan S.D., Yang M., Morgenthien E.A., Stauffer J.L. FVC variability in patients with idiopathic pulmonary fibrosis and role of 6-min walk test to predict further change. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.02151-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu X., Kim G.H., Salisbury M.L., et al. Computed tomographic biomarkers in idiopathic pulmonary fibrosis. The future of quantitative analysis. Am J Respir Crit Care Med. 2019;199(1):12–21. doi: 10.1164/rccm.201803-0444PP. [DOI] [PubMed] [Google Scholar]
- 87.Walsh S.L.F., Mackintosh J.A., Calandriello L., et al. Deep learning-based outcome prediction in progressive fibrotic lung disease using high-resolution computed tomography. Am J Respir Crit Care Med. 2022;206(7):883–891. doi: 10.1164/rccm.202112-2684OC. [DOI] [PubMed] [Google Scholar]
- 88.Ley B., Ryerson C.J., Vittinghoff E., et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 89.Wells A.U., Desai S.R., Rubens M.B., et al. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med. 2003;167(7):962–969. doi: 10.1164/rccm.2111053. [DOI] [PubMed] [Google Scholar]
- 90.Ward K., Spurr L., Goldman N.R., et al. Patient eligibility for anti-fibrotic therapy in idiopathic pulmonary fibrosis can be altered by use of different sets of reference values for calculation of FVC percent predicted. Respir Med. 2016;120:131–133. doi: 10.1016/j.rmed.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 91.Cortes-Telles A., Forkert L., O’Donnell D.E., Morán-Mendoza O. Idiopathic pulmonary fibrosis: new insights to functional characteristics at diagnosis. Can Respir J. 2014;21(3):e55–e60. doi: 10.1155/2014/825606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wells A.U. Forced vital capacity as a primary end point in idiopathic pulmonary fibrosis treatment trials: making a silk purse from a sow's ear. Thorax. 2013;68(4):309–310. doi: 10.1136/thoraxjnl-2012-202640. [DOI] [PubMed] [Google Scholar]
- 93.Prasse A., Probst C., Bargagli E., et al. Serum CC-chemokine ligand 18 concentration predicts outcome in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179(8):717–723. doi: 10.1164/rccm.200808-1201OC. [DOI] [PubMed] [Google Scholar]
- 94.Adegunsoye A., Alqalyoobi S., Linderholm A., et al. Circulating plasma biomarkers of survival in antifibrotic-treated patients with idiopathic pulmonary fibrosis. Chest. 2020;158(4):1526–1534. doi: 10.1016/j.chest.2020.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jenkins R.G., Simpson J.K., Saini G., et al. Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: an analysis from the prospective, multicentre PROFILE study. Lancet Respir Med. 2015;3(6):462–472. doi: 10.1016/S2213-2600(15)00048-X. [DOI] [PubMed] [Google Scholar]
- 96.van der Sar I.G., Moor C.C., Vellekoop B.P., Wijsenbeek M.S. Predicting treatment response in patients with interstitial lung disease using electronic nose technology. Eur Respir J. 2022;60(Suppl 66):345. [Google Scholar]
- 97.Moor C.C., Oppenheimer J.C., Nakshbandi G., et al. Exhaled breath analysis by use of eNose technology: a novel diagnostic tool for interstitial lung disease. Eur Respir J. 2021;57(1) doi: 10.1183/13993003.02042-2020. [DOI] [PubMed] [Google Scholar]
- 98.van der Sar I.G., Wijbenga N., Nakshbandi G., et al. The smell of lung disease: a review of the current status of electronic nose technology. Respir Res. 2021;22(1):246. doi: 10.1186/s12931-021-01835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yokoyama A., Kondo K., Nakajima M., et al. Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology. 2006;11(2):164–168. doi: 10.1111/j.1440-1843.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 100.Aloisio E., Braga F., Puricelli C., Panteghini M. Prognostic role of Krebs von den Lungen-6 (KL-6) measurement in idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Clin Chem Lab Med. 2021;59(8):1400–1408. doi: 10.1515/cclm-2021-0199. [DOI] [PubMed] [Google Scholar]
- 101.Wakamatsu K., Nagata N., Kumazoe H., et al. Prognostic value of serial serum KL-6 measurements in patients with idiopathic pulmonary fibrosis. Respir Investig. 2017;55(1):16–23. doi: 10.1016/j.resinv.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 102.Jiang Y., Luo Q., Han Q., et al. Sequential changes of serum KL-6 predict the progression of interstitial lung disease. J Thorac Dis. 2018;10(8):4705–4714. doi: 10.21037/jtd.2018.07.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Collard H.R., Calfee C.S., Wolters P.J., et al. Plasma biomarker profiles in acute exacerbation of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2010;299(1):L3–L7. doi: 10.1152/ajplung.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ohshimo S., Ishikawa N., Horimasu Y., et al. Baseline KL-6 predicts increased risk for acute exacerbation of idiopathic pulmonary fibrosis. Respir Med. 2014;108(7):1031–1039. doi: 10.1016/j.rmed.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 105.Bonella F., Ohshimo S., Boerner E., Guzman J., Wessendorf T.E., Costabel U. American Thoracic Society; 2015. Serum KL-6 levels correlate with response to pirfenidone in idiopathic pulmonary fibrosis. C42 SEARCHIN' for a cure: new ild treatments. American Thoracic Society international conference abstracts. [Google Scholar]
- 106.Chien J.W., Richards T.J., Gibson K.F., et al. Serum lysyl oxidase-like 2 levels and idiopathic pulmonary fibrosis disease progression. Eur Respir J. 2014;43(5):1430–1438. doi: 10.1183/09031936.00141013. [DOI] [PubMed] [Google Scholar]
- 107.Tzouvelekis A., Herazo-Maya J.D., Slade M., et al. Validation of the prognostic value of MMP-7 in idiopathic pulmonary fibrosis. Respirology. 2017;22(3):486–493. doi: 10.1111/resp.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Khan F.A., Stewart I., Saini G., Robinson K.A., Jenkins R.G. A systematic review of blood biomarkers with individual participant data meta-analysis of matrix metalloproteinase-7 in idiopathic pulmonary fibrosis. Eur Respir J. 2022;59(4) doi: 10.1183/13993003.01612-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Han M.K., Zhou Y., Murray S., et al. Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir Med. 2014;2(7):548–556. doi: 10.1016/S2213-2600(14)70069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chioccioli M., Roy S., Newell R., et al. A lung targeted miR-29 mimic as a therapy for pulmonary fibrosis. EBioMedicine. 2022;85 doi: 10.1016/j.ebiom.2022.104304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oak S.R., Murray L., Herath A., et al. A micro RNA processing defect in rapidly progressing idiopathic pulmonary fibrosis. PLoS One. 2011;6(6) doi: 10.1371/journal.pone.0021253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ryu C., Sun H., Gulati M., et al. Extracellular mitochondrial DNA is generated by fibroblasts and predicts death in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2017;196(12):1571–1581. doi: 10.1164/rccm.201612-2480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scott M.K.D., Quinn K., Li Q., et al. Increased monocyte count as a cellular biomarker for poor outcomes in fibrotic diseases: a retrospective, multicentre cohort study. Lancet Respir Med. 2019;7(6):497–508. doi: 10.1016/S2213-2600(18)30508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Karampitsakos T., Torrisi S., Antoniou K., et al. Increased monocyte count and red cell distribution width as prognostic biomarkers in patients with idiopathic pulmonary fibrosis. Respir Res. 2021;22(1):140. doi: 10.1186/s12931-021-01725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kreuter M., Lee J.S., Tzouvelekis A., et al. Monocyte count as a prognostic biomarker in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2021;204(1):74–81. doi: 10.1164/rccm.202003-0669OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jenkins G., Maher T.M., Cottin V., et al. Effect of nintedanib on blood biomarkers in patients with IPF in the INMARK trial. Eur Respir J. 2019;54(suppl 63) [Google Scholar]
- 117.Gui X., Qiu X., Xie M., et al. Prognostic value of serum osteopontin in acute exacerbation of idiopathic pulmonary fibrosis. BioMed Res Int. 2020;2020 doi: 10.1155/2020/3424208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oldham J.M., Allen R.J., Lorenzo-Salazar J.M., et al. PCSK6 and survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2023;207(11):1515–1524. doi: 10.1164/rccm.202205-0845OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Allen R.J., Oldham J.M., Jenkins D.A., et al. Longitudinal lung function and gas transfer in individuals with idiopathic pulmonary fibrosis: a genome-wide association study. Lancet Respir Med. 2023;11(1):65–73. doi: 10.1016/S2213-2600(22)00251-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tzouvelekis A., Herazo-Maya J.D., Ryu C., et al. S100A12 as a marker of worse cardiac output and mortality in pulmonary hypertension. Respirology. 2018;23(8):771–779. doi: 10.1111/resp.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Richards T.J., Kaminski N., Baribaud F., et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185(1):67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stuart B.D., Lee J.S., Kozlitina J., et al. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: an observational cohort study with independent validation. Lancet Respir Med. 2014;2(7):557–565. doi: 10.1016/S2213-2600(14)70124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Newton C.A., Batra K., Torrealba J., et al. Telomere-related lung fibrosis is diagnostically heterogeneous but uniformly progressive. Eur Respir J. 2016;48(6):1710–1720. doi: 10.1183/13993003.00308-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oldham J.M., Kumar D., Lee C., et al. Thyroid disease is prevalent and predicts survival in patients with idiopathic pulmonary fibrosis. Chest. 2015;148(3):692–700. doi: 10.1378/chest.14-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.O'Dwyer D.N., Armstrong M.E., Trujillo G., et al. The toll-like receptor 3 L412F polymorphism and disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2013;188(12):1442–1450. doi: 10.1164/rccm.201304-0760OC. [DOI] [PubMed] [Google Scholar]
- 126.Oldham J.M., Ma S.F., Martinez F.J., et al. TOLLIP, MUC5B, and the response to N-acetylcysteine among individuals with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2015;192(12):1475–1482. doi: 10.1164/rccm.201505-1010OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kotsianidis I., Nakou E., Bouchliou I., et al. Global impairment of CD4+CD25+FOXP3+ regulatory T cells in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179(12):1121–1130. doi: 10.1164/rccm.200812-1936OC. [DOI] [PubMed] [Google Scholar]
- 128.Reilkoff R.A., Peng H., Murray L.A., et al. Semaphorin 7a+ regulatory T cells are associated with progressive idiopathic pulmonary fibrosis and are implicated in transforming growth factor-β1-induced pulmonary fibrosis. Am J Respir Crit Care Med. 2013;187(2):180–188. doi: 10.1164/rccm.201206-1109OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Surolia R., Li F.J., Wang Z., et al. 3D pulmospheres serve as a personalized and predictive multicellular model for assessment of antifibrotic drugs. JCI Insight. 2017;2(2) doi: 10.1172/jci.insight.94088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Herazo-Maya J.D., Noth I., Duncan S.R., et al. Peripheral blood mononuclear cell gene expression profiles predict poor outcome in idiopathic pulmonary fibrosis. Sci Transl Med. 2013;5(205) doi: 10.1126/scitranslmed.3005964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van der Vis J.J., Prasse A., Renzoni E.A., et al. MUC5B rs35705950 minor allele associates with older age and better survival in idiopathic pulmonary fibrosis. Respirology. 2023;28(5):455–464. doi: 10.1111/resp.14440. [DOI] [PubMed] [Google Scholar]
- 132.Dudbridge F., Allen R.J., Sheehan N.A., et al. Adjustment for index event bias in genome-wide association studies of subsequent events. Nat Commun. 2019;10(1):1561. doi: 10.1038/s41467-019-09381-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Spagnolo P., Oldham J.M., Jones M.G., Lee J.S. Personalized medicine in interstitial lung diseases. Curr Opin Pulm Med. 2017;23(3):231–236. doi: 10.1097/MCP.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 134.Juan Guardela B.M., Sun J., Zhang T., et al. 50-gene risk profiles in peripheral blood predict COVID-19 outcomes: a retrospective, multicenter cohort study. EBioMedicine. 2021;69 doi: 10.1016/j.ebiom.2021.103439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.DePianto D.J., Chandriani S., Abbas A.R., et al. Heterogeneous gene expression signatures correspond to distinct lung pathologies and biomarkers of disease severity in idiopathic pulmonary fibrosis. Thorax. 2015;70(1):48–56. doi: 10.1136/thoraxjnl-2013-204596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gülden E., Vudattu N.K., Deng S., et al. Microbiota control immune regulation in humanized mice. JCI Insight. 2017;2(21) doi: 10.1172/jci.insight.91709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kinder B.W., Brown K.K., McCormack F.X., et al. Serum surfactant protein-A is a strong predictor of early mortality in idiopathic pulmonary fibrosis. Chest. 2009;135(6):1557–1563. doi: 10.1378/chest.08-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Takahashi H., Fujishima T., Koba H., et al. Serum surfactant proteins A and D as prognostic factors in idiopathic pulmonary fibrosis and their relationship to disease extent. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1109–1114. doi: 10.1164/ajrccm.162.3.9910080. [DOI] [PubMed] [Google Scholar]
- 139.Greene K.E., King T.E., Jr., Kuroki Y., et al. Serum surfactant proteins-A and -D as biomarkers in idiopathic pulmonary fibrosis. Eur Respir J. 2002;19(3):439–446. doi: 10.1183/09031936.02.00081102. [DOI] [PubMed] [Google Scholar]
- 140.Barlo N.P., van Moorsel C.H., Ruven H.J., Zanen P., van den Bosch J.M., Grutters J.C. Surfactant protein-D predicts survival in patients with idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26(2):155–161. [PubMed] [Google Scholar]
- 141.Clynick B., Jo H.E., Corte T.J., et al. Circulating RNA differences between patients with stable and progressive IPF. Eur Respir J. 2020;56 doi: 10.1183/13993003.02058-2019. [DOI] [PubMed] [Google Scholar]
- 142.Song J.W., Do K.H., Jang S.J., Colby T.V., Han S., Kim D.S. Blood biomarkers MMP-7 and SP-A: predictors of outcome in idiopathic pulmonary fibrosis. Chest. 2013;143(5):1422–1429. doi: 10.1378/chest.11-2735. [DOI] [PubMed] [Google Scholar]
- 143.Yu G., Tzouvelekis A., Wang R., et al. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med. 2018;24(1):39–49. doi: 10.1038/nm.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Okamoto M., Izuhara K., Ohta S., Ono J., Hoshino T. Ability of periostin as a new biomarker of idiopathic pulmonary fibrosis. Adv Exp Med Biol. 2019;1132:79–87. doi: 10.1007/978-981-13-6657-4_9. [DOI] [PubMed] [Google Scholar]
- 145.Korthagen N.M., van Moorsel C.H., Barlo N.P., et al. Serum and BALF YKL-40 levels are predictors of survival in idiopathic pulmonary fibrosis. Respir Med. 2011;105(1):106–113. doi: 10.1016/j.rmed.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 146.Kahloon R.A., Xue J., Bhargava A., et al. Patients with idiopathic pulmonary fibrosis with antibodies to heat shock protein 70 have poor prognoses. Am J Respir Crit Care Med. 2013;187(7):768–775. doi: 10.1164/rccm.201203-0506OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Neighbors M., Cabanski C.R., Ramalingam T.R., et al. Prognostic and predictive biomarkers for patients with idiopathic pulmonary fibrosis treated with pirfenidone: post-hoc assessment of the CAPACITY and ASCEND trials. Lancet Respir Med. 2018;6(8):615–626. doi: 10.1016/S2213-2600(18)30185-1. [DOI] [PubMed] [Google Scholar]
- 148.Saini G., Porte J., Weinreb P.H., et al. αvβ6 integrin may be a potential prognostic biomarker in interstitial lung disease. Eur Respir J. 2015;46(2):486–494. doi: 10.1183/09031936.00210414. [DOI] [PubMed] [Google Scholar]
- 149.Jenkins G., Noth I., Selman M., et al. Effects of nintedanib on markers of epithelial damage in subjects with IPF: data from the INMARK trial. Eur Respir J. 2020;56(Suppl 64):5187. [Google Scholar]
- 150.Maher T.M., Stowasser S., Nishioka Y., et al. Biomarkers of extracellular matrix turnover in patients with idiopathic pulmonary fibrosis given nintedanib (INMARK study): a randomised, placebo-controlled study. Lancet Respir Med. 2019;7(9):771–779. doi: 10.1016/S2213-2600(19)30255-3. [DOI] [PubMed] [Google Scholar]
- 151.Yoshikawa T., Otsuka M., Chiba H., et al. Surfactant protein A as a biomarker of outcomes of anti-fibrotic drug therapy in patients with idiopathic pulmonary fibrosis. BMC Pulm Med. 2020;20(1):27. doi: 10.1186/s12890-020-1060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ikeda K., Shiratori M., Chiba H., et al. Serum surfactant protein D predicts the outcome of patients with idiopathic pulmonary fibrosis treated with pirfenidone. Respir Med. 2017;131:184–191. doi: 10.1016/j.rmed.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 153.Ikeda K., Chiba H., Nishikiori H., et al. Serum surfactant protein D as a predictive biomarker for the efficacy of pirfenidone in patients with idiopathic pulmonary fibrosis: a post-hoc analysis of the phase 3 trial in Japan. Respir Res. 2020;21(1):316. doi: 10.1186/s12931-020-01582-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.https://classic.clinicaltrials.gov/ct2/show/NCT04300920
- 155.d'Alessandro M., Bergantini L., Cameli P., et al. Serum concentrations of KL-6 in patients with IPF and lung cancer and serial measurements of KL-6 in IPF patients treated with antifibrotic therapy. Cancers. 2021;13(4) doi: 10.3390/cancers13040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nakamura M., Okamoto M., Fujimoto K., et al. A retrospective study of the tolerability of nintedanib for severe idiopathic pulmonary fibrosis in the real world. Ann Transl Med. 2019;7(12):262. doi: 10.21037/atm.2019.05.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Peljto A.L., Kim J., Xu H., et al. A polygenic risk score for idiopathic pulmonary fibrosis and interstitial lung abnormalities. B93 breakthroughs in ild: translation to the bedside. 2023. p. A4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Juan-Guardela B.M., Herazo-Maya J.D. Immunity, ciliated epithelium, and mortality: are we ready to identify idiopathic pulmonary fibrosis endotypes with prognostic significance? Chest. 2022;161(6):1440–1441. doi: 10.1016/j.chest.2022.02.018. [DOI] [PubMed] [Google Scholar]
- 159.De Sadeleer L.J., Verleden S.E., Schupp J.C., et al. BAL transcriptomes characterize idiopathic pulmonary fibrosis endotypes with prognostic impact. Chest. 2022;161(6):1576–1588. doi: 10.1016/j.chest.2021.12.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.https://clinicaltrials.gov/ct2/show/NCT03312400
- 161.Townsley D.M., Dumitriu B., Liu D., et al. Danazol treatment for telomere diseases. N Engl J Med. 2016;374(20):1922–1931. doi: 10.1056/NEJMoa1515319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Karampitsakos T., Papaioannou O., Katsaras M., Sampsonas F., Tzouvelekis A. Interstitial lung diseases and the impact of gender. Clin Chest Med. 2021;42(3):531–541. doi: 10.1016/j.ccm.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 163.Antoniou K.M., Tsitoura E., Vasarmidi E., et al. Precision medicine in idiopathic pulmonary fibrosis therapy: from translational research to patient-centered care. Curr Opin Pharmacol. 2021;57:71–80. doi: 10.1016/j.coph.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 164.Thannickal V.J., Antony V.B. Is personalized medicine a realistic goal in idiopathic pulmonary fibrosis? Expert Rev Respir Med. 2018;12(6):441–443. doi: 10.1080/17476348.2018.1464913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Maher T.M., Avram C., Bortey E., et al. Nalbuphine tablets for cough in patients with idiopathic pulmonary fibrosis. NEJM Evid. 2023;2(8) doi: 10.1056/EVIDoa2300083. [DOI] [PubMed] [Google Scholar]