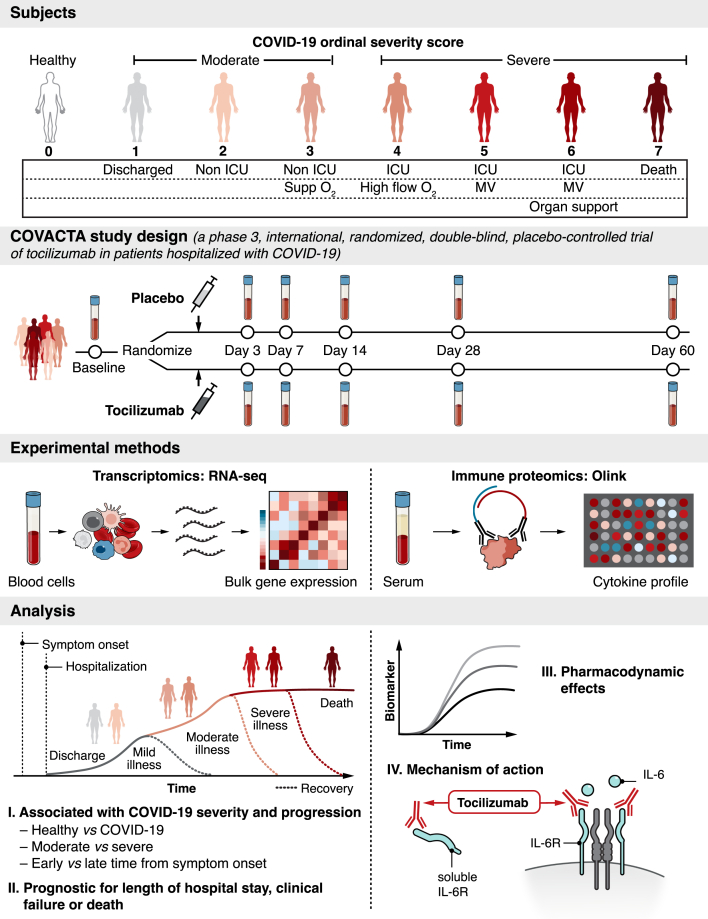
Figure 1.
Serum protein and whole blood RNA profiling from hospitalized patients with COVID-19 enrolled in the COVACTA study at baseline and during treatment with tocilizumab
Disease severity was assessed at baseline and after dosing using a 7-category ordinal scale. At baseline, “moderate” COVID-19 was defined as an ordinal scale score <4 and “severe” COVID-19 was defined as ordinal scale score ≥4. The present analysis included an external control group using peripheral blood from healthy adults. Patients enrolled in COVACTA received a single intravenous tocilizumab infusion (8 mg/kg) or placebo in addition to standard care. Serum and peripheral whole blood collected from COVACTA patients at baseline (before dosing) and up to 60 days after dosing were profiled by proteomics (Olink) and transcriptomics (RNA-Seq) assays. Key immune aberrations associated with COVID-19, time from symptom onset, and disease severity were identified at baseline and assessed for prognostic association with time to hospital discharge/ready for discharge, mortality, and time to clinical failure. To understand mechanism of action, the pharmacodynamic effect of tocilizumab and the response of severity associated biomarkers to tocilizumab treatment were assessed in longitudinal samples. ICU, intensive care unit; IL-6R, interleukin 6 receptor; MV, mechanical ventilation.