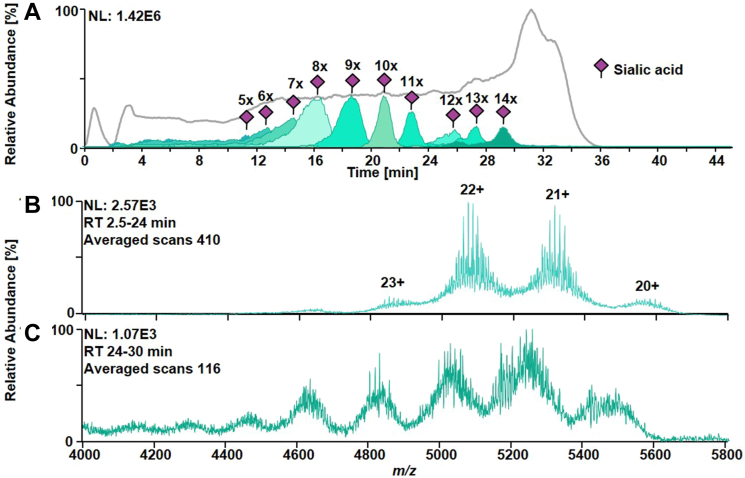
Fig. 3.
SAX-HPLC-MS analysis of intact glycoforms of Myozyme.A, in gray, the total ion current chromatogram (TICC) obtained upon elution of glycoforms in a range of approximately 30 min is reported. The extracted ion chromatograms (XICs) of different ions of r-hGAA glycoforms carrying an increasing number of sialic acids are represented in green shading. Increased chromatographic retention is correlated with a higher degree of sialylation of glycoforms. B, raw mass spectrum associated to the retention time window from 2.5 to 24 min (410 averaged scans). A charge state distribution from 20 to 23 positive charges indicates the preservation of a quasi-native state of the glycoforms during the chromatographic separation. C, raw mass spectrum associated to the retention time window from 24 to 30 min (116 averaged scans). Despite the acidic conditions of the mobile phases (≈ pH 4–3) in this range of the gradient, only slight denaturation of the protein was observed as indicated by an increased number of charge states. MS, mass spectrometry; r-hGAA, recombinant human acid alpha-glucosidase; SAX, strong anion exchange.