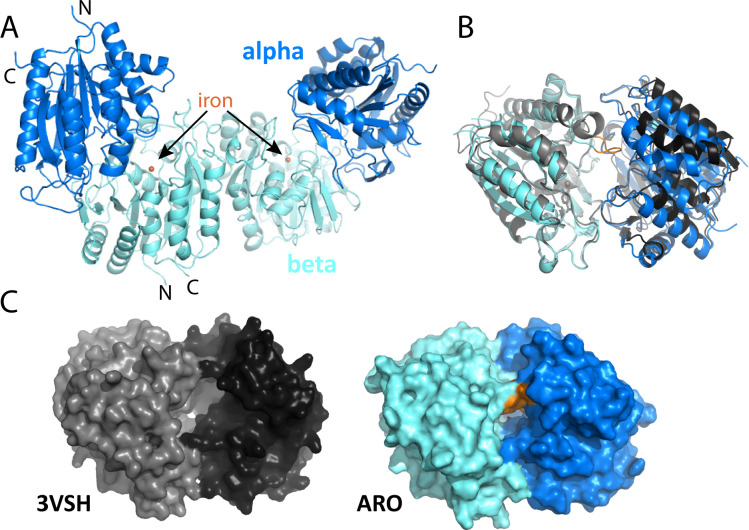
Fig 4.
Structure overview of M. rosaria ARO dioxygenase and comparison with the non-BMC-associated homolog from Comamonas testosteroni. (A) Structure of the biological dimer with iron active site in the beta subunit marked. N-terminal and C-terminal residues (D3 and H278, respectively, for alpha; T30 and G322 for beta subunits) are marked. (B) Alignment of ARO dioxygenase with the homologous structure (with aligned beta subunits); a large difference is observed in a loop at residues N184-Y189. (C) Comparison of surface view of the two structures reveals differences in active site accessibility.