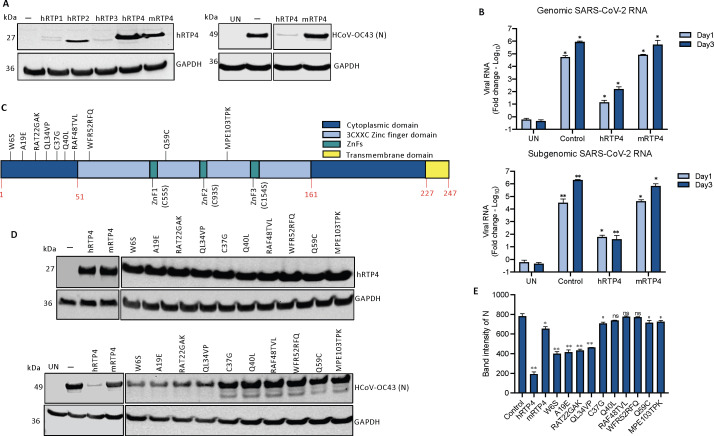
FIG 5 .
mRTP4 does not restrict coronavirus replication. (A) Western blot analysis of lysates from CHME3 cells following transfection of FLAG-tagged RTP4 protein constructs, including hRTP1, hRTP2, hRTP3, hRTP4 (WT), and mRTP4 or empty vector control. The blots were probed with anti-FLAG tag and GAPDH antibodies (left). CHME3 cells expressing the indicated hRTP4 and mRTP4 constructs were infected with HCoV-OC43 at an MOI of 0.5. After, 48 h, the cells were lysed the viral N protein was quantified on immunoblot analysis using GAPDH as a loading control (right). (B) ACE2.CHME3 cells were infected with SARS-CoV-2 at an MOI of 0.1 at 1 and 3 dpi. RNA levels were quantified by qRT-PCR. The data were normalized to GAPDH and expressed as the fold-change relative to uninfected cells. The data are averages of three biological replicates. (C) Illustration depicting RTP4 human to murine amino acid residue point mutations. (D) CHME3 cells were transfected with expression vectors for murine RTP4 and indicated human to mRTP4 residue mutants along with the hRTP4 or empty vector control. Expression levels were quantified on an immunoblot probed with anti-FLAG and GAPDH antibodies (top). The transfected cells were infected with HCoV-OC43 at an MOI of 0.5. After 48 h, the viral N protein was quantified by immunoblot analysis with GAPDH as a loading control (bottom). (E) Band intensities from the immunoblot analysis for the HCoV-OC43 N protein expression levels are plotted. The experiment was repeated twice with similar results (*P < 0.05, **P < 0.01).