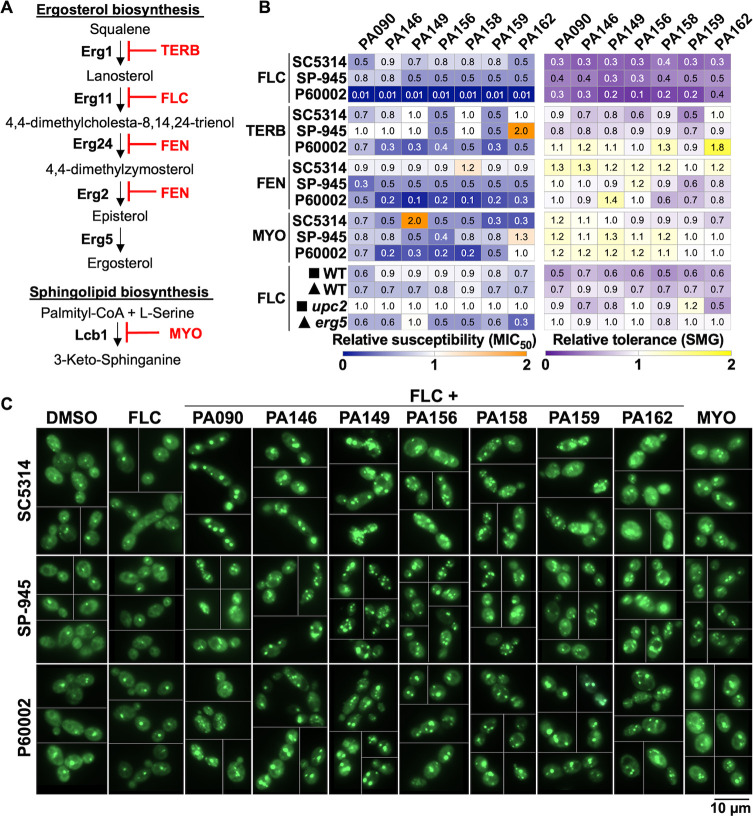
Fig 9.
1,4-Benzodiazepines (1,4-BZDs) potentiate inhibitors of ergosterol and sphingolipid biosynthesis. (A) Terbinafine (TERB) and fenpropimorph (FEN) target ergosterol biosynthesis via Erg1 and Erg24/Erg2, respectively, while myriocin (MYO) targets sphingolipid biosynthesis via Lcb1. (B) Changes in FLC, TERB, FEN, or MYO susceptibility (MIC50) and tolerance (SMG) when Candida albicans isolates were treated with combinations of 1,4-BZDs (at 100 µM) and serial dilutions of fluconazole (FLC) (0 to 128 µg/mL), TERB (0 to 32 µg/mL), FEN (0 to 32 µg/mL), or MYO (0 to 128 µg/mL), relative to DMSO controls. FLC data are included for reference. Deletion mutants of Upc2, the central regulator of ergosterol synthesis genes, and Erg5, a cytochrome P450 enzyme catalyzing the last step in ergosterol biosynthesis, were also tested with FLC and 1,4-BZDs; symbols adjacent to strain names denote their corresponding lineage/WT controls, all constructed in the SC5314 background. All values are shown relative to DMSO controls and indicate the average from three to four biological replicates. (C) C. albicans cells stained with the BODIPY 493/503 dye following 4-hour incubation with either DMSO, FLC (128 µg/mL), MYO (32 µg/mL), or combinations of FLC (128 µg/mL) and 1,4-BZDs (100 µM). Images show representative cells for the respective conditions, at 60X magnification. The relative dilution and distribution of yeast cells on the slides necessitated the creation of composite images to ensure the best representation of the phenotypes. Scale bar, 10 µm.