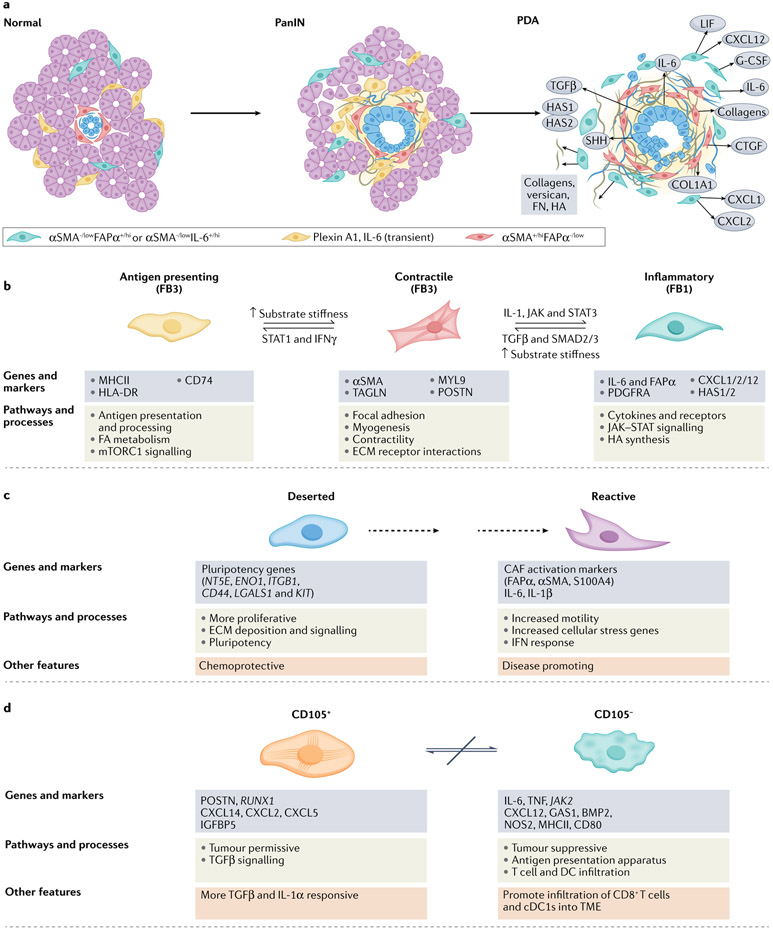
Fig. 2 ∣. Mesenchymal cell lineages and diversity in pancreatic cancer.
Our understanding of fibroblast heterogeneity is beginning to progress beyond a simple binary designation of ‘quiescent’ versus ‘activated’ states. Instead, phenotypically, functionally and spatially distinct cancer-associated fibroblast (CAF) subsets are emerging. Mesenchymal cells, derived from both resident pancreatic stellate cells (PSCs) and bone marrow-derived stem cells, respond to cues from the epithelium and infiltrating immune cells and evolve coordinately in a developing pancreatic neoplasm. a, Although the detailed classification schemes and lists of markers for these subsets continue to evolve with advancing technologies, the integrated information from several early studies suggested two broad classes of CAF subsets68-70,75,77. One subset of CAFs appear to have a more contractile phenotype, participate in juxtacrine signalling with tumour epithelial cells through close apposition and typically overexpress αSMA. Although signalling and metabolic support (see below) together with the onset of increased tension associated with matrix stiffening can promote transformation of early epithelia, the sustained activation, increasing fibrosis and increased contractility of these adjacent myofibroblasts may also act as a counter and constrain invasion and migration of epithelial cells in later stages of disease. A separate set of fibroblasts may be more removed from epithelial cells, express lower levels of αSMA and higher levels of FAPα, and possess an immunosuppressive phenotype characterized by secretion of IL-6, CXCL12 and other key inflammatory chemokines and cytokines. These two subtypes appear to be interconvertible depending on exposure to key signals from the tumour epithelial cells, such as TGFβ and IL-1, as well as mechanical cues from the underlying matrix. Another study described a third population in mouse pancreatic ductal adenocarcinoma (PDA) that expresses plexin A1 and IL-6 and exists transiently in the normal pancreas and in a pancreas with pre-invasive ductal neoplasms but is lost in the setting of invasive disease51. The spatial distribution of these functional subtypes may therefore be defined in part by gradients of signalling molecules (indicated by background yellow shading) from the tumour epithelium. b, The relatively recent ability to perform massive parallel transcriptional analyses of single cells followed by iterative unsupervised clustering analyses has provided unprecedented resolution of the subtypes, numbers and activities of cells in normal tissues, tumours and other disease states. Several such analyses have been performed on human pancreatic cancers and genetically engineered mouse models (GEMMs) of the disease51-56. Each of these studies has broadly confirmed the existence of two functional classes of fibroblasts, with the possibility of a third, while also providing new insights into their evolution during disease progression. Elyada et al. compared analyses of GEMMs with human pancreatic cancers and normal adjacent tissues from resected specimens52. Their studies on human CAFs confirmed their prior designations of inflammatory and myofibroblastic subtypes, with the former expressing immune and redox regulatory genes and hyaluronan synthases, and the latter expressing genes involved in contractility and the mesenchymal state. The initial analyses of mouse CAFs recapitulated the human findings; however, to further increase the sensitivity of the analysis, the investigators performed negative selection followed by selection for podoplanin (PDPN) expression (as a pan-CAF marker) to enrich for CAFs from their single-cell suspension. These studies permitted the identification of a potential third subtype of CAFs characterized by expression of MHCII genes, including Cd74, but not co-stimulatory molecules, and were designated antigen-presenting CAFs. Interestingly, this population also expressed mesothelin. The possibility that fibroblasts might take up and/or express mesothelin on their cell surface and present it to T cells was raised in an earlier study of adoptive T cell therapy in a KrasLSL–G12D/+;Trp53LSL–R175H/+;Cre(KPC) GEMM of PDA in which not only tumour epithelial cells but also some fibroblasts appeared to undergo T cell-mediated apoptosis209. On returning to their human dataset, the authors could discern a population of CAFs expressing modest levels of MHCII genes, not as a separate subcluster but, rather, that were more diffusively distributed across the other two CAF clusters and, perhaps to a greater extent, with inflammatory CAFs. Imaging mass cytometry of human pancreatic tumours confirmed the presence of cells co-expressing HLA-DR and CD74. Hosein et al.51 analysed principally a KrasLSL–G12D/+; Ink4aflox/flox;Cre (KIIC) model, complemented with some analyses of KPC mice, and identified three transcriptionally distinct fibroblast subtypes (FB1, FB2 and FB3) in the normal mouse pancreas, of which two persisted through to invasive disease. The FB2 cluster expanded during progression to pre-invasive disease before disappearing altogether, leaving only the FB1 (inflammatory-like) and FB3 (myofibroblast-like) populations in roughly equal proportions in advanced disease. In their description of FB3 myofibroblasts, this subset also expressed MHCII, a mark of professional antigen-presenting cells (APCs), and genes associated with the mesothelial state51. Single-cell RNA sequencing complemented by cytometry time-of-flight (CyTOF) analyses of human and mouse specimens in a study by Zhang et al.55 was broadly consistent with the picture presented thus far. A subsequent study confirmed the mesothelial cell origin of the MHCII-expressing CAFs210 and found that the transition to an antigen-presenting CAF phenotype was induced by IL-1 and TGFβ, molecules also implicated in inducing inflammatory and myofibroblastic CAFs, respectively77. These CAFs also promoted the expansion of immunosuppressive regulatory T cells. How exactly these mesothelial cell-derived fibroblasts and dermatopontin-positive universal fibroblasts relate to each other, and to the respective PDA CAF populations they give rise to, remains to be clarified. c, A competing conceptualization and radical departure for understanding CAF diversity was instead rooted in distinct phenotypic and functional properties of CAFs isolated from two unique histopathological subdomains identified in resected human PDAs53. These subdomains, or sub-tumour microenvironments (subTMEs), were distinguished by their cellularity and ECM abundance: the ‘deserted’ subTME was paucicellular but had extensive ECM deposition, whereas ‘reactive’ regions were populated with more fibroblasts, endothelial cells and immune cells, including evidence of direct contact between T cells and tumour epithelial cells. Deserted CAFs were more proliferative and expressed genes associated with pluripotency; reactive CAFs were more motile and expressed signatures associated with EMT, activation and inflammation. The relative abundance of these subTMEs varied with respect to disease evolution and response to therapy. Deserted subTMEs increased after exposure to cytotoxic chemotherapy and were therefore described as being chemoresistant, whereas reactive subTMEs became sparse; conditioned media from deserted CAFs were chemoprotective for patient-derived organoids (PDOs) in culture. Reactive subTMEs increased overall as disease progressed, and conditioned media from reactive CAFs increased PDO proliferation. These results therefore suggest that the phenotypic behaviours of the malignant epithelium in PDA are defined, or at least strongly influenced, by factors from their adjoining CAF subpopulations. If this classification endures further validation, it could have profound implications for prognosis and for informing treatment course. d, A more simplified depiction of CAF heterogeneity in PDA emerged from mass cytometry of 19 individual pancreatic cancers from the KPC GEMM of PDA (and was later confirmed in other models and human PDAs)54. These analyses found that PDPN, CD90, desmin and CD63 were expressed on most CAFs and that other common markers, including αSMA, PDGFRα and/or PDGFRβ, ICAM1, integrin α5 and CD73, showed graded expression across several subclusters, revealing a spectrum of phenotypic states. Expression of αSMA and expression of PDGFRα were inversely related: αSMAhiPDGFRαlow CAFs and αSMAlowPDGFRαhi CAFs largely formed separate groups of clusters, and were most closely reminiscent of myofibroblastic CAFs and inflammatory CAFs, respectively. Expression of CD105 (part of the TGFβ receptor complex) cleanly delineated two distinct CAF populations, each again containing both contractile and inflammatory signatures (that is, αSMA and PDGFRα showed graded expression across both populations). CD105+ CAFs were described as tumour permissive on the basis of subcutaneous co-injection experiments with tumour epithelial cells, and CD105− CAFs were tumour suppressive in a manner that depended on an intact adaptive immunity. Similar populations were found in human PDAs and in normal pancreatic tissue, defining CD105 as a key cell surface determinant of distinct fibroblast lineages in human and mouse pancreas. CD105+ CAFs tended to be more intra-acinar, and CD105− CAFs tended to be more inter-acinar. Each population manifested a great deal of plasticity in terms of responses to various stimuli, with each class capable of engaging the same signalling nodes, although with distinct outputs. The two populations were not interconvertible, which is consistent with them representing distinct lineages, albeit of currently unknown provenance. This newly presented conceptualization of two fixed lineages with dynamic plasticity within each lineage and distinct abilities to influence disease trajectory could also have enormous implications for patient management. PanIN, pancreatic intraepithelial neoplasias.