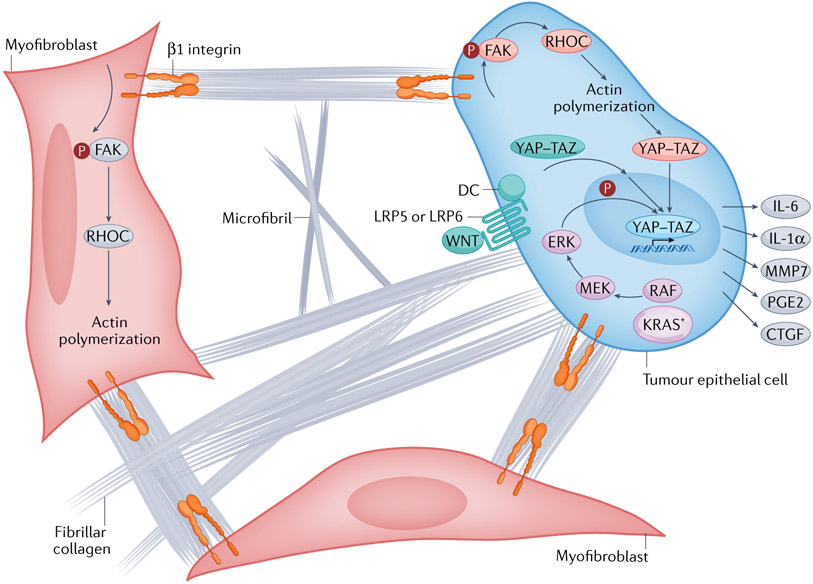
Fig. 5 ∣. Activation of intracellular mechanisms of force transduction in pancreatic cancer by tensile loading of the collagen network.
Surface binding of fibrillar collagen to β1 integrins of tumour epithelial cells and myofibroblasts activates a cascade of focal adhesion complex maturation, RHO–RHO-associated kinase (ROCK) activation, phosphorylation of myosin light chain 2 (MLC2; not shown), actin polymerization and myosin-induced contractility. This feeds back to further enhance focal adhesion formation until intracellular force generation matches the traction forces applied by the extracellular matrix (ECM) (that is, the cell pulls back). In this manner, matrix stiffness and intracellular contractility are tuned to maintain tensional homeostasis. Increased fibrosis (fibrillar collagen deposition), increased collagen and microfibril crosslinking, and increased swelling pressures (from hydrated hyaluronan (HA)) can each augment the applied load and transmitted force through the surface-bound integrins. This concerted mechanism of signal transduction also suggests there may be additional targets for interventions to disrupt the feedforward loop, decrease interstitial pressures, decrease force generation and decrease mechanosignalling. The downstream consequence of this mechanosignalling is nuclear translocation of the Yes-associated protein (YAP)–transcriptional co-activator with PDZ-binding motif (TAZ) transcriptional complex, which, together with TEA domain family member (TEAD), drives a unique transcriptional programme of cell-autonomous and non-cell-autonomous behaviours, including a secretory programme promoting ECM remodelling and the influx of various immune cell subsets. The oncoprotein KRAS further shapes the YAZ–TAZ–TEAD transcriptional repertoire by activating MAPK signalling to induce distinct phosphorylation events on the DNA-binding complex. Finally, aberrant WNT signalling leads to the dissolution of the destruction complex (DC) and release of bound YAP–TAZ, which can then translocate to the nucleus. In this manner, numerous signalling pathways and biophysical stimuli converge to amplify YAP–TAZ signalling, perhaps providing the ability to even substitute for KRAS-G12D signalling once a sufficient threshold of activity is achieved. CTGF, connective tissue growth factor (also known as CCN2); FAK, focal adhesion kinase; IL, interleukin; KRAS*, mutant KRAS; LRP, low-density lipoprotein receptor-related protein; MMP7, matrix metalloproteinase 7; PGE2, prostaglandin E2.