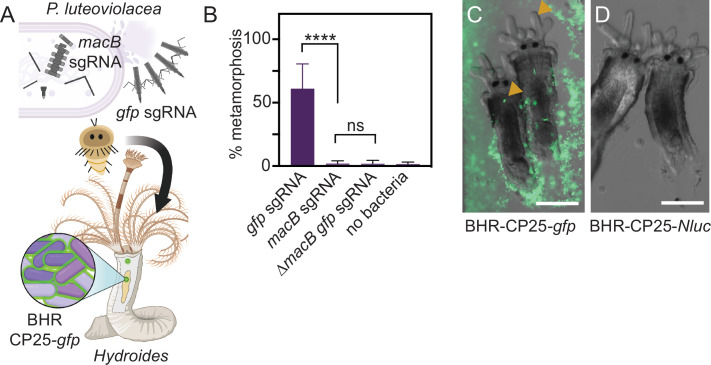
Fig 3.
Functional knockdown of MACs and visualization of P. luteoviolacea during the tubeworm-microbe interaction. (A) Schematic depicting P. luteoviolacea and the production of MACs, which induce tubeworm metamorphosis. CRISPRi single-guide RNA (sgRNA) targeting the macB MACs baseplate gene prevents MACs from assembling, rendering the bacterium unable to induce metamorphosis. Cells that produce intact MACs are able to induce tubeworm metamorphosis. A strong fluorescent reporter strain (BHR-CP25-gfp) enabled visualization of live tubeworm-bacteria interactions. (B) Bar graph representing biofilm metamorphosis assays with P. luteoviolacea carrying a CRISPRi plasmid targeting macB or gfp and Hydroides tubeworms. A P. luteoviolacea ∆macB strain with a sgRNA targeting gfp and a treatment without bacteria (no bacteria) were included as controls. Biofilm concentrations were made with cells at OD600 0.1. Bars plotted show the average of 12 replicates, performed across three independent experiments. Each well contained 20–40 worms. Error bars indicate standard deviations. Statistical significance between treatments was determined by Dunn’s multiple comparisons test (N = 12). (C and D) Merged fluorescence and DIC micrographs of Hydroides elegans juveniles imaged 24 h after the competent larvae were exposed to inductive biofilms of P. luteoviolacea containing plasmids with (C) CP25-gfp or (D) CP25-NLuc. Strains containing NLuc plasmids were used as a negative control to account for autofluorescence. Yellow arrows show accumulation of fluorescent bacteria in the Hydroides juvenile pharynx. Scale bar is 100 µm.