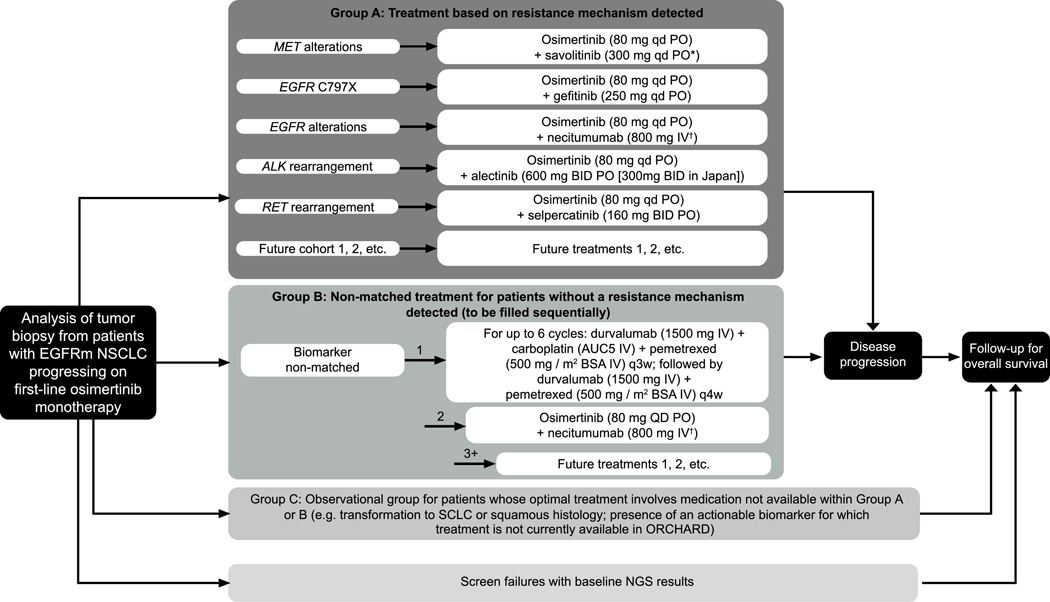
Figure 1.
ORCHARD Study Design
*Following a protocol amendment, all newly enrolled patients with MET alterations will receive savolitinib 300 mg qd, replacing the previous weight-based dosing regimen (300 mg for patients weighing ≤55 kg at screening, or 600 mg once daily for patients >55 kg at screening).
†On days 1 and 8 of each 3-week cycle.
Group A: Patients who are positive for protocol-determined biomarker; Group B: patients without an available protocol-determined biomarker match, allocated sequentially, once first cohort cap has been reached, the next cohort allocation will begin; Group C: observational cohort, treated in accordance with local practice. Most treatments will be provided by the sponsor. In some participating countries, pemetrexed and carboplatin are sourced locally.
Abbreviations: ALK = anaplastic lymphoma kinase; AUC= area under curve; BID = twice daily; EGFR = epidermal growth factor; EGFRm = EGFR-tyrosine kinase inhibitor sensitizing mutation-positive; IV = intravenous; NGS = next-generation sequencing; NSCLC = non-small cell lung cancer; PO = orally; q3(4)w = once every 3(4)-week cycle; QD = once daily; SCLC = small cell lung cancer.