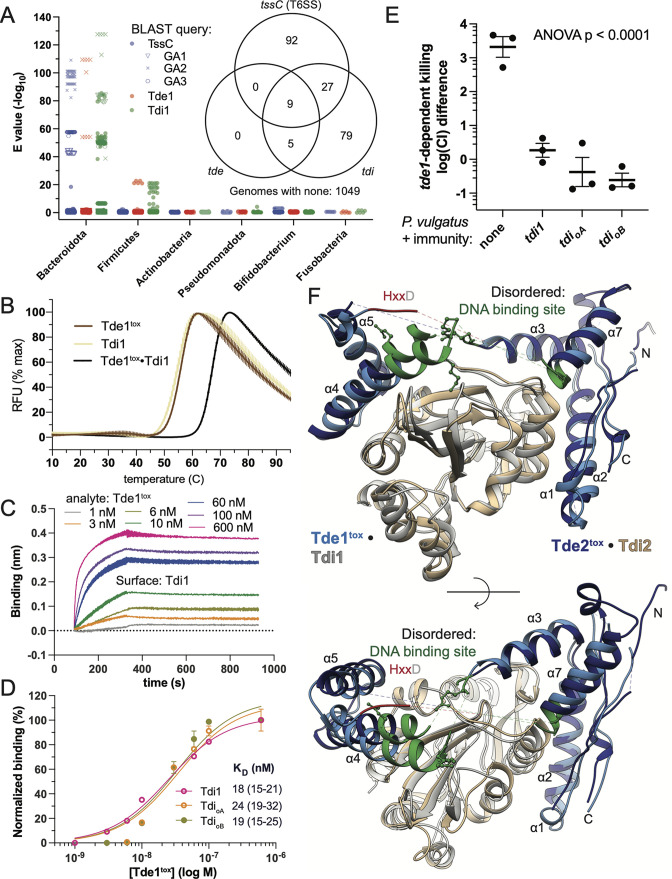
FIG 3.
Cognate and orphan immunity proteins protect against T6SS-mediated attack by inducing a conformational shift in Tde1 to disrupt the DNA binding and active sites. (A) Query of Tde1, Tdi1, and representative T6SS structural protein (TssC) against a collection of ~1,200 human intestinal commensal genomes (40) with BLAST revealed predominant distribution of homologs within Bacteroidota. TssC homologs from previously described genetic architectures (GA1-3) cluster together (15). Tde1, but not Tdi1 homologs are exclusively in GA2 T6SS. Several Firmicutes harbor tde/tdi pairs not associated with T6SS. Immunity encoding genes were more abundant than tde. (inset) A Venn diagram illustrates that all identified tde1 homologs were accompanied by tdi. tde/tdi pairs were associated with a T6SS apparatus in 9 Bacteroidota and 5 Firmicutes. However, tdi genes were more frequently encountered than tde in both phyla, indicating presence of orphan immunity genes. (B) Tde1tox • Tdi1 exhibited higher thermal stability (melting temperature 67°C) than either component alone (55–55.5°C) in SYBR orange thermal melt experiments. (C and D) Biolayer interferometry demonstrated comparable equilibrium binding affinities of Tde1tox for Tdi1, as well as two homologous orphan immunity proteins (KD 18–24 nM). (E) Expression of Tdi1, as well as two orphan immunity proteins from diverse Bacteroidota protect P. vulgatus ATCC 8482 against tde1-dependent attack by P. vulgatus MSK 16.10. (F) Crystal structures of two homologous Tdetox (blues) and Tdi (gray, tan) complexes demonstrate a splitting of Ntox15 into two subdomains. The subdomains are linked by the DNA binding site and the HxxD motif, which are partially disordered in the crystal structures (dotted lines). The predicted DNA binding site is green, and basic residues required for high affinity DNA interaction represented as sticks. There is high structural similarity among the homologs, indicating a conserved mode of interaction.