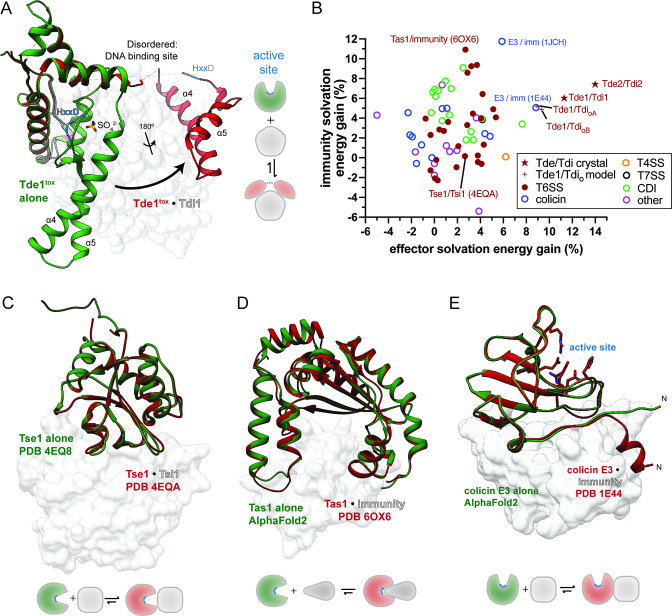
FIG 4.
Effector fold disruption is a new immunity mechanism among polymorphic toxins. (A) The Tde1tox alone structure (red) is superimposed on the Tde1tox/Tdi1 complex structure. Upon immunity binding, the split subdomains of Tde1tox undergo a relative ~90° hinge motion and ~180° rotation. The DNA binding site (including helix α6) and the active site (HxxD yellow) are disrupted by the conformational shift. (B) Solvation energy gains of effector/immunity interface formation as percentages of monomer solvation energy were calculated with PDBePISA (41). Included structural models with PDB accession and PubMed IDs are listed in Table S2. Tde1tox/orphan immunity calculations are derived from comparative homology models based on the Tde1tox/Tdi1 structure. (C) The “capping” mechanism with non-disruptive steric occlusion of the effector active site is typified by the Pseudomonas aeruginosa T6SS-assocated peptidoglycan hydrolase Tse1/Tsi1. (D) Several T6SS and other polymorphic toxin/immunity interactions involve insertion of the immunity protein into a pre-formed effector active site crevice (“plugging”), typified by P. aeruginosa (P)ppApp synthetase Tas1/immunity. A predicted model of Tas1 alone, supported by an experimental structure of homolog RelQ (not shown, PDB 5DEC), indicates lack of large conformational shift in the effector. (E) A structure of colicin E3 RNAse exhibits engagement of immunity at an “exosite” separate from the enzymatic active site (42). Unlike Tde1tox/Tdi1, large effector conformational shifts are not predicted.