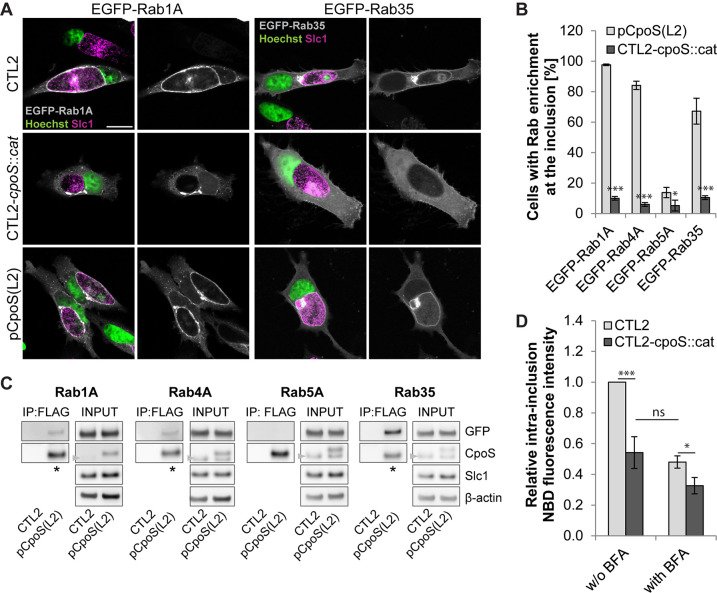
Fig 5.
CpoS mediates the recruitment of Rab GTPases and modulates membrane trafficking. (A) Localization of EGFP-tagged Rab1A and Rab35 in infected HeLa cells (5 IFU/cell, 24 hpi, confocal, scale = 20 µm). (B) Percentage of infected HeLa cells with enrichment of EGFP-Rab proteins at the inclusion (5 IFU/cell, 24 hpi). BFA (3 µg/mL) added at 18 hpi [mean ± SD, n = 3 (at least 250 cells per condition), Student’s t-test]. (C) Co-IP confirms the interaction between CpoS and Rab GTPases. HeLa cells were infected (10 IFU/cell; CTL2 or CTL2-cpoS::cat/pCpoS(L2)-FLAG) and transfected with EGFP-Rab expression plasmids. CpoS-FLAG was precipitated from lysates at 26 hpi. Samples were analyzed by western blot analysis (* indicates visible Co-IP of EGFP-tagged Rab proteins). Note that the CpoS-specific antibody detects both endogenous and FLAG-tagged CpoS, yet also a non-specific band [also seen in uninfected cells (not shown)] that overlaps with the band of endogenous CpoS (arrowheads). (D) Reduced ceramide acquisition by CpoS-deficient strain. At 14 hpi, infected HeLa cells (5 IFU/cell) were treated with NBD C6-ceramide, in the presence or absence of BFA (3 µg/mL). At 21 hpi, the average intra-inclusion NBD fluorescence intensity was determined and is displayed relative to the average intensity observed in CTL2 inclusions in the absence of BFA (mean ± SD, n = 3, one-way ANOVA).