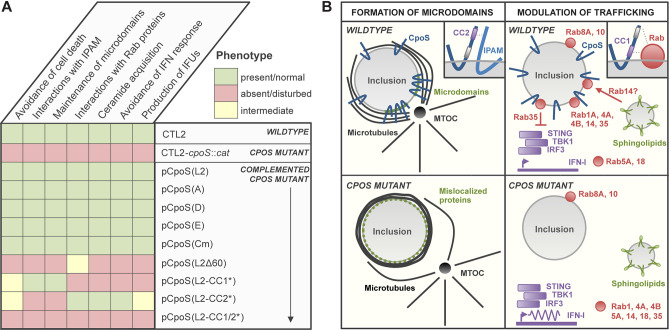
Fig 7.
Graphical summary of discoveries reported in this study. (A) Overview of the phenotypes of the cpoS mutant and the capabilities of distinct CpoS homologs and variants to complement the observed defects. (B) Illustration summarizing our two main findings, that is, a role of CpoS in the maintenance of inclusion microdomains (left), and a role of CpoS-Rab35 GTPase interactions in the suppression of the host cellular type I IFN response (right). In brief, we showed that CpoS itself is enriched at inclusion microdomains and that its absence disrupts the microdomain localization of multiple other inclusion-associated proteins, as well as the cytoskeletal architecture at the inclusion. At the molecular level, this function of CpoS in microdomains depends on an intact CC2 motif in CpoS, a motif also required for CpoS–IPAM interactions, strongly suggesting a role for IncInc interactions in the maintenance of inclusion microdomains. In addition, we found that a functional CC1 motif in CpoS and in part also additional amino acids at the C-terminus of CpoS are required for CpoS to interact with host Rab GTPases and to recruit these proteins to the inclusion. Significantly, CpoS–Rab interactions not only play a role in the modulation of host membrane trafficking, for example, sphingolipid transport to inclusions, but also in the suppression of the host cellular type I IFN response. Specifically, we revealed a role for Rab35 in the dampening of STING/TBK1/IRF3 signaling in cells infected with C. trachomatis.