Abstract
Bone has long been considered as a silent organ that provides a reservoir of calcium and phosphorus, traditionally. Recently, further study of bone has revealed additional functions as an endocrine organ connecting systemic organs of the whole body. Communication between bone and other organs participates in most physiological and pathological events and is responsible for the maintenance of homeostasis. Here, we present an overview of the crosstalk between bone and other organs. Furthermore, we describe the factors mediating the crosstalk and review the mechanisms in the development of potential associated diseases. These connections shed new light on the pathogenesis of systemic diseases and provide novel potential targets for the treatment of systemic diseases.
Keywords: bone, crosstalk, endocrine organs, osteocalcin, osteokines, receptor activator for nuclear factor-κ B ligand
Introduction
Bone is a living and dynamic organ, which coordinately processes bone formation by osteoblasts and bone resorption by osteoclasts, respectively. Osteoblasts develop from mesenchymal stem cells, and osteoclasts are large multinucleated cells that develop from hematopoietic stem cells. Another bone cell type, osteocytes, are the most abundant cells, comprising 95% of bone cells and regulating osteoclasts and osteoblasts to maintain bone balance [1]. These cells are influenced by numerous local and systemic factors and produce factors that participate in systemic body regulation. The mutual regulation among these cells contributes to maintaining bone homeostasis.
The endocrine system plays crucial roles in the maintenance of whole organism physiology. Traditionally, bone has long been considered as an inertia organ that provides the storage for calcium and phosphate to support the body. Recently, bone revealed extra-skeletal functions [2] and is redefined as an active endocrine organ. The crosstalk between bone and other organs is essential to whole-body homeostasis, coordinating the activity of organs and guaranteeing the proper physiological functions of organs. Communication between bone and other organs is increasingly recognized as a critical way to maintain homeostasis and disease adaptation [3]. In this review, we will present an overview of the crosstalk between bone and other organs, including muscle, brain, immune system, blood vessel, pancreas, kidney, liver and gonad (Figure 1).
Figure 1:
Crosstalk between bone and other organs. There is a tight connection between bone and other organs, including muscle, brain, immune system, blood vessel, pancreas, kidney, liver and gonad.
Crosstalk between bone and muscle
The musculoskeletal system is comprised of muscle and bone. Bone provides attachment sites for muscle, and skeletal muscle imparts a force on the bone to facilitate locomotion of the organism. Compared with bones, muscle was recognized as an endocrine organ earlier. Bone and muscle interact to maintain their structures and functions, and their anatomic and physiological connections have been considered to transmit that the mechanical forces applied to muscle to the skeleton to promote bone formation, historically [4, 5].
The coexistence of osteoporosis and sarcopenia has been recently considered as a syndrome termed ‘osteosarcopenia’, which is common in older age and is associated with significant morbidity and mortality [6]. However, it is unclear whether one condition precedes the other or if the conditions are linked with energy demand to facilitate bone formation [7]. Muscle and bone can also secrete and receive common factors to regulate their metabolism, as well as that of the whole body [7]. When contraction occurs, muscle is most likely producing osteocyte viability factors, which lost with aging [8]. Therefore, it is essential to identify the muscle factors that protect osteocytes and the bone factors that maintain muscle function to design therapeutics for the prevention and treatment of osteoporosis and sarcopenia [8].
Recent studies have shown that biochemical communication also exists between muscle and bone in addition to mechanical interactions through the endocrine system mediated by myokines (derived from myocytes) and osteokines (derived from bone cells) as shown in Figure 2. In this section, we will discuss the bidirectional effects of myokines and osteokines on bone and muscle metabolism.
Figure 2:
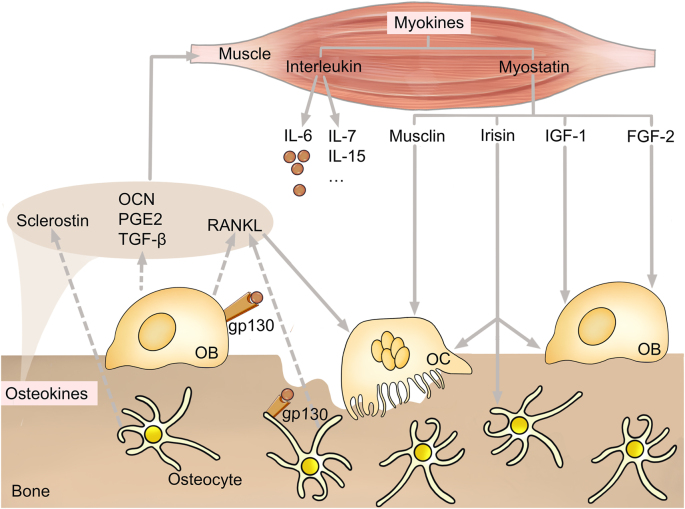
Crosstalk between bone and muscle. Osteokines produced and released by bone and myokines produced and released by muscle together maintain the crosstalk between bone and muscle. Osteokines secreted by osteoblast (OB) and osteocyte (such as sclerostin, osteocalcin prostaglandin E2, transforming growth factor-β and receptor activator for nuclear factor-κ B ligand) affect muscle metabolism. Myokines secreted by muscle include interleukin and myostatin. IL-6 regulates OB and osteocyte by binding to the IL-6 receptor gp130. Myostatins (such as musclin, irisin, insulin like growth factor-1 and fibroblast growth factor 2) also affect osteocyte, OB and osteoclast. OCN, osteocalcin; PGE2, prostaglandin; TGF-β, transforming growth factor-β; RANKL, receptor activator for nuclear factor-κ B ligand; IL, include interleukin; IGF-1, insulin like growth factor-1; FGF-2, fibroblast growth factor 2; OC, OB and osteoclast.
Bone to muscle
The number of bone‐derived factors, namely, osteokines, continues to increase, including osteocalcin (OCN), sclerostin, prostaglandin E2 (PGE2), transforming growth factor-β (TGF-β) and receptor activator for nuclear factor-κ B ligand (RANKL). Herein, we describe the effects of these factors on muscle in this review.
OCN, an osteoblast-derived molecule encoded by the bone gamma-carboxyglutamate protein gene, is so important in life processes. It has been reported that OCN levels increase during physical activity and meanwhile, the level of OCN decreases during aging [9]. Administration of exogenous OCN increases the exercise capacity of 3-month-old mice and restores the exercise capacity of 9-, 12- and 15-month-old mice by promoting glucose absorption and stimulating catabolism in skeletal muscle [10]. Undercarboxylated OCN has a close relationship with the muscle regulation of insulin sensitivity [11]. In addition, OCN promotes the synthesis of interlukin (IL)-6 [12], which in turn promotes adaptation to exercise by stimulating OCN in bone.
Sclerostin is encoded by the SOST gene. It is secreted by osteocytes and suppresses bone formation via the canonical Wnt/β‐catenin pathway [13]. The absence of sclerostin caused by sclerostin antibody or SOST gene intervention technology increases bone mass [14] and may further induce sclerosteosis [15]. Instead, the level of sclerostin is reduced after muscle or bone loading [16, 17].
PGE2 is essential for efficacious skeletal muscle-specific stem cell function, augmenting regeneration and strength by interacting with its receptor EP4 [18]. PGE2 activates the β-catenin pathway via the stimulation of PI3K in osteocytes in response to fluid shear stress [19], and PGE2 accelerates C2C12 myoblast proliferation [20]. PGE2 signaling ameliorates muscle atrophy and rejuvenates muscle function. Thus, the PGE2-degrading enzyme 15-PGDH may be a potential therapeutic target for preventing sarcopenia [21].
TGF‐β is another muscle regulator that is mainly derived from bone and is stored within the mineralized bone matrix. Mice treated with TGF‐β reduces the production of specific muscle force, but the muscle mass remains unchanged [22]. Moreover, TGF‐β causes muscle weakness in the setting of osteolytic cancer through an increase in muscle oxidative stress and calcium mishandling [8, 23].
RANKL is a key mediator of osteoclast formation, function, and survival [24]. Overexpression of RANKL induces bone loss, which is associated with impairment of muscle function and strength. RANKL receptor RANK deletion in muscle prevents muscle atrophy and dysfunction induced by denervation [25]. Anti-RANKL treatment (such as denosumab) protects against skeletal muscle dysfunctions while enhancing bone mechanical properties [26]. Moreover, denosumab significantly increases appendicular lean mass and improves handgrip strength, suggesting that bone and muscle have a tight connection and that denosumab could represent a novel therapeutic approach for sarcopenia [27].
Muscle to bone
Muscle influences bone metabolism mainly via myokines, including interleukins and myostatin. Understanding the functional role and signaling pathways of myokines, particularly as they relate to exercise, may reveal new therapeutic targets to promote bone health.
One of the most significant interleukins is IL-6. While, IL-6 derived from muscle is stimulated by exercise [28] and generally acts as an anti-inflammatory compound and increases glucose uptake and sensitivity [29, 30]. The roles of IL-6 in bone are negative to some extent by binding to its soluble receptor, gp130. IL-6 drives osteoclastogenesis via promoting the release of RANKL by osteoblasts, osteocytes and leukocytes and increasing the expression of RANK in osteoclast to reveal a net resorptive effect [31]. Mechanically loaded myotubes promote osteoclasts formation through the secretion of IL‐6 [32]. IL‐7 and IL-15, abundantly secreted by muscle, are also widely regarded as osteoclastogenic factors [8, 33].
Myostatin is a secreted growth and differentiation factor that belongs to the TGF-β superfamily [34] and has been clearly defined as a negative regulator of muscle and bone [35, 36]. Myostatin elicits osteoclastogenesis and reduces bone formation [35, 37]. Suppressing myostatin in the osteogenic differentiation of bone marrow stem cells induces the expression of osteogenic growth factors, such as insulin like growth factor-1 (IGF-1), leading to increasing osteoblast proliferation and accelerating bone formation [37]. Inhibition of the soluble myostatin decoy receptor ActRIIB, which is expressed on the cell membrane of osteoblasts, increases bone formation [38, 39]. Some studies have proposed that myostatin stimulates the production of sclerostin, RANKL, Dickkopf, and Wnt signaling pathway inhibitor 1 (DKK1) [40] and accelerates the absorption of osteocyte-derived exosomes by osteoblasts, leading to reducing osteoblastogenesis [41]. In contrast, myostatin acts as a positive regulator of osteoclast formation by activating the RANKL/SMAD2/nuclear factor of activated T cells (NFATc1) signaling pathway [42].
Irisin is a peptide produced by proteolytic cleavage of fibronectin type III domain-containing protein 5, a transmembrane protein localized in skeletal muscle mediated by exercise [43]. Irisin was initially regarded as a hormone that induces thermogenesis in adipose tissue [43], but recently, a potent ability to modulate the bone turnover has been revealed. Several studies have shown that irisin is positively associated with bone mineral status [44]. It inhibits osteoclast differentiation by suppressing the RANKL/NFATc1 and nuclear factor-κB (NF-κB) pathways [45] and promotes osteoblast differentiation by upregulating Nrf2 and inhibiting the NLRP3 inflammasome [46]. In contrast, irisin increases sclerostin expression in osteocytes to induce bone resorption and deletion of irisin completely blocks the ovariectomy-induced trabecular bone loss [47]. Thus, the role of irisin in the skeleton is quite complex and requires further study.
Musclin is a novel skeletal muscle-derived secretory factor [48]. Its protein sequence is similar to that of osteocrin, a protein expressed in osteoblasts which disappears after birth [49]. Thus, musclin may play important roles in bone. As we speculated, musclin enhances C-type natriuretic peptide receptor signaling by targeting NPR3, a C-type natriuretic peptide clearance receptor, for degradation. Musclin mRNA expression has been linked to insulin induced Akt and forkhead box O1 transcription factor signaling pathways, leading to osteoclastogenesis [50].
IGF-1 and fibroblast growth factor 2 (FGF-2), two well-known osteogenic factors that are abundant in homogenized muscle tissue [12, 51], are localized at the muscle-bone interface [52]. IGF-1 is predominantly synthesized by the liver and is expressed in multiple extrahepatic tissues, including bone and skeletal muscle. IGF-1 is necessary for osteoblasts, osteoclasts and osteocytes and maintains the balanced interaction between osteoblasts and osteoclasts [53, 54]. It positively regulates osteoblast function through the Wnt/β-catenin pathway [55] and stimulates the survival of osteocytes and osteoblasts [56]. On the other hand, IGF-1 induces osteoclastogenesis by enhancing RANKL synthesis [57]. FGF-2 is localized to the muscle–bone interface and could be a bone formation inducer factor released by muscle [52, 58]. Moreover, FGF-2 targets sclerostin in bone and myostatin in muscle to reduce the damage of glucocorticoids on musculoskeletal degradation, suggesting a tight connection between bone and muscle [59].
Crosstalk between bone and brain
The brain has long been known as the main coordinator for the activities and hormonal secretion of other organs. An increasing number of studies have revealed that the reciprocal effects on the brain are dependent on peripheral organs, especially the bone. The central nervous system (CNS) directly regulates bone tissues via efferent neural connections. In particular, osteoporosis has been associated with multiple brain dysfunctions as well as major neurodegenerative diseases, including stroke [60], Alzheimer’s disease (AD) [61] and Parkinson’s disease [61]. Bone cells contain the innervation and receptors for several neural peptides, and the neural tracts from the bone marrow link the central nervous system. Similarly, skeletal disorders are associated with changes in brain activity, potentially shared with interconnecting molecular mechanisms to some extent.
Bone to brain
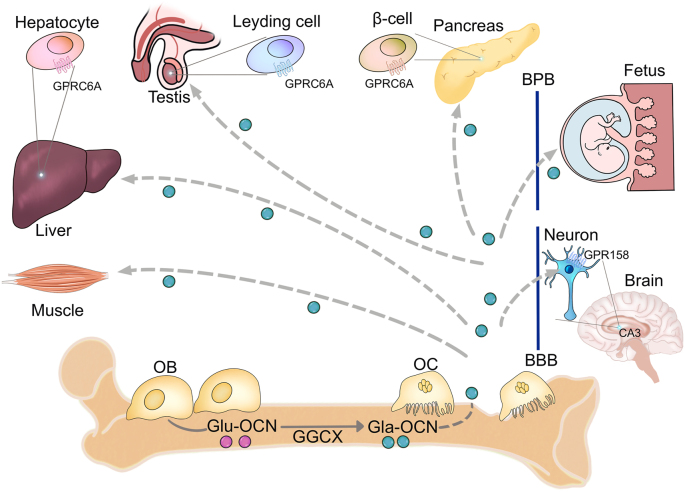
Undercarboxylated, bioactive OCN, initially considered as a regulator in bone remodeling [62] and metabolism [63], and promotes testosterone synthesis of the testis and the fertility of male mice [64, 65]. Inchoately, OCN is C-carboxylated (Gla-OCN) and secreted by osteoblasts into the bone extracellular matrix [66]. Then, carboxylase γ-glutamyl carboxylase decarboxylates Gla-OCN into undercarboxylated active OCN (Glu-OCN). With the help of osteoclasts during the progress of bone resorption, the affinity of OCN for the bone matrix decreases and Gla-OCN is promoted to entry into the circulation to act as a hormone for other organs [67, 68] (Figure 3). Glu-OCN regulates energy metabolism by binding to the receptor GPRC6A in the testis, liver, pancreas and muscle [69]. While in brain, GPR158 acts as a receptor for OCN, which expresses in the CA3 region of the hippocampus in pyramidal neurons (Figure 3) in part through inositol 1,4,5-trisphosphate and brain-derived neurotrophic factor [70]. Kandel further found that RbAp48 is a critical component of GPR158/OCN signaling. Activation of the OCN/GPR158 pathway increases the expression of RbAp48 rescues age-related memory loss [71].
Figure 3:
Endocrine roles of osteocalcin. Carboxylase γ-glutamyl carboxylase facilitates the posttranslational modification of C-carboxylated OCN into undercarboxylated active OCN. Bone resorption of osteoclast decreases the OCN affinity for the bone matrix and promotes the entry of Gla-OCN into the circulation to act as a hormone by binding to its receptor. Gla-OCN crosses the blood-brain barrier and the placenta to affect the development of brain and memory and crosses the blood-placenta barrier to affect the spatial learning-like behavior of fetus. In addition, Gla-OCN regulates the metabolism of muscle, liver, testis and pancreas. OCN, osteocalcin; GGCX, Carboxylase γ-glutamyl carboxylase; Gla-OCN, C-carboxylated; Glu-OCN, undercarboxylated active OCN; OC, osteoclast; BBB, blood-brain barrier; BPB, blood-placenta barrier.
OCN is not expressed in any part of the brain, but it can cross the blood-brain barrier and bind with brain regions, such as the hippocampus, the ventral tegmental area, the substantia nigra, and the brainstem to regulate neurotransmitters content. As a result, maternal OCN prevents depression and anxiety and crosses the placenta to influence the brain development of fetus during embryogenesis (Figure 3). The above phenomenon may be explained by the fact that the content of monoamine neurotransmitter increased with sufficient OCN, including norepinephrine, dopamine and serotonin [72]. Deficiency of OCN impairs the functions of memory in the offspring as evidenced by anatomical defects in the hippocampal region observed with an increase in the number of apoptotic cells [73].
Similar to OCN, lipocalin 2, another osteoblast-derived mediator [74], is a glycoprotein that regulates energy metabolism by mediating insulin secretion and improving glucose tolerance and insulin sensitivity. It can also cross the blood-brain barrier to activate the anorexigenic pathway by binding to the melanocortin 4 receptor in the hypothalamus [75]. Lipocalin 2 KO mice showed increased gonadal fat weight, total fat mass and body weight and exogenous lipocalin 2 exerted a sustained anorexigenic effect, leading to a decreased fat mass, body weight and body-weight gain [75].
In addition, osteocyte-specific sclerostin also plays important roles in brain function. Sclerostin binds to the Lrp4/5/6 to further antagonize Wnt signaling [76], resulting in reducing bone formation and promoting bone resorption [77] and further associated with the pathophysiology of brain diseases, such AD [78].
Bone and bone marrow are regarded as a single unit or two perspectives of one organ. In addition to the factors secreted by bone, bone marrow-derived cells can also enter the systemic circulation and migrate into the brain [79]. It has been reported that bone marrow-derived microglia-like cells improve cognitive impairment by restricting Aβ plaque formation and supporting Aβ plaque clearance in amyloid pathology [80].
Brain to bone
The sympathetic nervous system functions by releasing norepinephrine, which can activate α-adrenergic receptors (ARs) in presynaptic cells and activate β-adrenergic receptors in postsynaptic cells. Sympathetic postganglionic neurons project to most tissues of the body, including bone [81]. Sympathetic periosteal fibers branch in the compact bone and bone marrow and secret factors, such as the sympathetic nerve markers: tyrosine hydroxylase [82], dopamine β-hydroxylase [83], neuropeptide Y and norepinephrine transporter [84]. The parasympathetic nervous system postsynaptic neurons mainly supply cholinergic terminals for excretory and reproductive functions. The neurotransmitter acetylcholine plays important roles in the functions of parasympathetic nervous system by activating muscarinic and nicotinic cholinergic receptors. The vesicular acetylcholine transporter [84] and choline acetyltransferase [85] have also been detected in bone. If we consider the skeleton in the dimension of the internal organs, it is easier to understand the skeletal innervation, especially the autonomic nerve innervation of the skeleton. The brain is a powerful regulator of skeletal homeostasis. The CNS regulates bone mass by the direct action of neurotransmitters or by acting as a mediator in mediating peripheral hormonal signals, such as leptin.
Leptin was first cloned in 1994 [86], and it is a vertebrate invention, suggesting that bone may be its major target [87]. On the one hand, leptin regulates appetite, energy expenditure and fertility regulated by brain. On the other hand, leptin links to bone remodeling: mice deficient in leptin (ob/ob) or its receptor (db/db) display a low bone mass phenotype [88, 89]. The function of leptin in bone formation through the hypothalamus reveals completely opposite effects. Leptin exerts a catabolic effect on bone mass via activation of the sympathetic nervous system [90]. Once leptin binds to the leptin receptor and activates the tyrosine kinase Jak2, resulting in the phosphorylation of the residues on the leptin receptor [91]. Next, the expression of tryptophan hydroxylase 2 decreases. Tryptophan hydroxylase 2 encodes the initial enzyme for the biosynthesis of serotonin, which in ventromedial neurons decreases the activity of sympathetic nervous system and reduces bone mass.
ARs belong to the G protein-coupled receptor superfamily. They regulate presynaptic sympathetic nerve terminal signals, release the neurotransmitter norepinephrine and participate in brain and peripheral nerves and in target tissues, such as bone. ARs consist of two subtypes, αARs and βARs. Both osteoblasts [92] and osteoclasts [93] express αAR. βARs may be the main AR that regulates the effects of sympathetic nerves on bone remodeling. βARs are subdivided into three groups: β1ARs, β2ARs, and β3ARs. Osteoblasts mainly express the β2AR subtype [84, 94, 95]. While β1ARs and β3ARs were found to be weakly expressed or even undetectable in osteoblasts and osteoclasts [96].
Additionally, silencing IL-1 receptor signaling in the central nervous system leads to very low skeletal vesicular acetylcholine transporter expression, acetylcholine levels, and low bone mass. These results suggest that the “central IL-1-parasympathetic-bone” axis antagonizes skeletal sympathetic tone and promotes bone mass accrual [97]. Semaphorin 3A is the first identified vertebral semaphorin and is characterized as a diffusible axonal chemorepellent that guarantees the growth and branching of axons into appropriate areas [98]. Currently, Semaphorin 3A is recognized to play important roles in bone remodeling. It regulates osteoclast differentiation by binding to neuropilin-1, and Semaphorin 3a KO mice exhibit severely low bone mass due to the increased numbers of osteoclasts and the decreased numbers of osteoblasts [99].
Crosstalk between bone and immune system
Osteoimmunology is an interdisciplinary research field that explores the shared molecules and interactions between the bone and immune systems. In 1972, Horton first reported the interaction between immune cells and bone cells and found that bacterial antigen-stimulated immune cells produce osteoclast activating factors [100]. In 2000, Arron and Choi proposed the term ‘osteoimmunology’ [101], which provides a conceptual bridge to understand the novel biological framework and a molecular basis for the exploration of therapies for diseases of bone and/or the immune system. The bone and immune systems share a variety of molecules, such as cytokines, chemokines, transcription factors and signaling molecules. Furthermore, accumulating evidence has shown that bone cells reciprocally regulate immune cells and hematopoiesis [102, 103]. Bone remodeling is closely connected with lymphohematopoietic homeostasis because the bone marrow is formed by trabecular bone structures that provide a solid niche for the maintenance and differentiation of hematopoietic stem cells (ancestors of blood lineage cells and osteoclasts) and mesenchymal stem cells (ancestors of chondrocytes, adipocytes and osteoblasts) [104]. Here, we summarize the recent advances in the regulation of immune cells by bone cells and the effects of the immune system on bone cells, and discuss the application of osteoimmunology in treating diseases.
Bone to immune system
Bone is not only a crucial element of the skeletal-locomotor system, but also functions as an immunological organ. Skin is a physical immune organ that acts as a barrier to protect us from harmful exposure to external and internal environments [105]. From an evolutionary point of view, bone originates as mineralization around the basal membrane of the throat or skin [106]. Bone is specific to vertebrates, whose evolution is dependent on exposure to sunlight and photosynthesis of vitamin D3 in the skin [107]. Voisin proposed that the skin langerhans cells and osteoclasts likely evolved from a common ancestor from a genetic and a functional point of view [108]. Besides, bone cells and immune cells share the same micromilieu in bone marrow, where hematopoietic stem cells and immune progenitor cells ultimately migrate during mammalian development [102, 109].
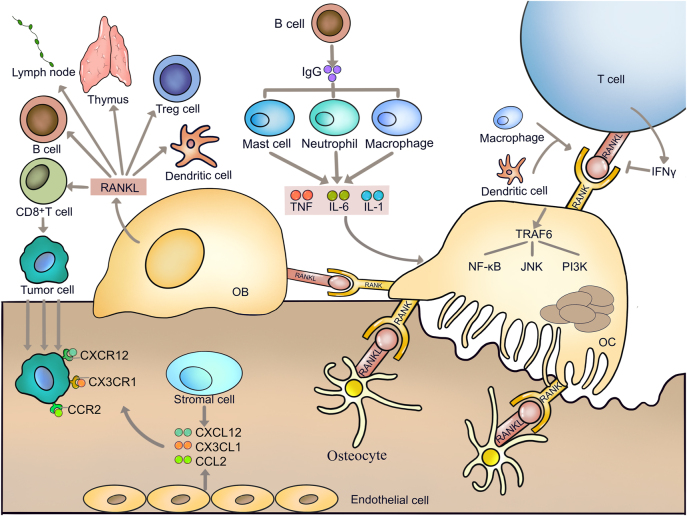
RANKL is one of the most important factors linking the two systems. It plays multiple roles in the immune system, including mediating immunological responses and immune organ development. RANKL was originally identified as a T-cell-derived cytokine that regulates the function of dendritic cells. Further studies show that RANKL secreted from osteoblasts and osteocytes participates in the maturation of dendritic cells [110, 111]. The studies of TNF receptor superfamily member (Tnfrsf)11a or tumor necrosis factor superfamily 11 KO mice reveal that the RANKL/RANK system is essential for the development of immune organs, such as the thymus (Figure 4). RANKL is essential for the development and function of the secondary lymphoid organs, where immune responses take place and lymph node is one such organ (Figure 4). RANKL on lymphoid tissue organizer cells and marginal reticular cells binds to RANK on lymphatic endothelial cells, resulting in the recruitment of macrophages [110, 112]. Additionally, RANKL shows immunosuppressive effects in the immunotherapies in cancers. It has been reported that treatment with denosumab in premenopausal early-stage breast cancer patients inhibits RANK pathway and increases tumor infiltrating lymphocytes and CD8+ T cells on the one hand [113]. On the other hand, RANKL shows the opposite effects on autoimmune diseases, including rheumatoid arthritis [114], experimental autoimmune encephalomyelitis [115] and Crohn’s disease [116]. Thus, the anti-RANKL treatment would be a potential strategy for autoimmune diseases. In addition, RANK on lymphatic endothelial cells is important for the retention of lymphoid tissue inducer cells in the lymph node anlagen [117]. Blockade of the RANKL signal by the RANK-Fc protein decreases the number of regulatory T (Treg) cells and promotes the differentiation of CD8+ T cells into cytotoxic T lymphocytes, leading to type 1 diabetes [118]. Lack of RANKL impairs B-cell development, reducing the immunoglobulin-secreting B cells (Figure 4) [119]. However, conditional knockout of Tnfrsf11a in B cells does not affect B-cell development or antibody production [120].
Figure 4:
Crosstalk between bone and immune system. Receptor activator for nuclear factor-κ B ligand is one of the most important factors linking bone and immune system. RANKL produced and released by osteoblast promotes osteoclastogenesis and regulates the function of B cells, T cells, Treg cells and dendritic cells. In addition, it affects the development of thymus and lymphoid organs. RANKL promotes the differentiation of CD8+ T cells into cytotoxic T lymphocytes to suppress cancer development. Stromal cells and endothelial cells in bone marrow produce C-X-C motif chemokine ligand 12, CX3C chemokine ligand 1, and C-C motif chemokine ligand 2 to bind to their receptors on cancer cells and attract cancer cell migration to bone. In turn, T cells, B cells, dendritic cells and macrophages also promote the osteoclastogenesis. RANKL, Receptor activator for nuclear factor-κ B ligand; OB, osteoblast; CXCL12, C-X-C motif chemokine ligand 12; CX3CL1, CX3C chemokine ligand 1; CCL2, C-C motif chemokine ligand 2.
Osteoblasts participate in the differentiation of T cells and B cells in the bone marrow through other factors, such as the Notch ligand delta-like 4. Osteoblasts express Notch ligand delta-like 4 to support the development of T cell progenitors [121]. Deletion of C-X-C motif chemokine ligand 12 from osterix-expressing stromal cells in osteoblasts reduces the number of B-lymphoid progenitors [122]. In addition, bone marrow stem cells secrete IL-7 to promote T cells and B cells development [123].
Immune system to bone
Immune cells play a critical role in postmenopausal osteoporosis. Ovariectomy could not induce cortical and trabecular bone loss in T cell deficient nude mice, suggesting that T cells exert protective effects on bone structure [124, 125]. Specifically, both CD4+ T cells, including type helper (Th)1, Th2, Th17 and Treg subsets, and CD8+ T cells play key roles in ovariectomy-induced bone loss [126]. The IgG immune complex secreted by B cells regulates osteoclast differentiation via promoting TNF, IL-1 and IL-6 secreted by innate immune cells, such as mast cells, neutrophils and macrophages [127] (Figure 4). Th17 cells are the main cells causing osteoclastogenesis by producing higher levels of IL-17, RANKL and TNF-α and lower levels of IFN-γ [128, 129]. While Treg cells suppress the effector functioning of Th17 cells via their production of IL-4, IL-10 and TGF-β1 and suppress bone loss by inhibiting the differentiation of monocytes into osteoclasts [130].
Butyrate stimulates bone formation via Treg cells and regulates Wnt10b production by CD8+ T cells [131]. In addition, Treg cells play a role in bone formation by directly promoting the differentiation of osteoblasts [132]. On the contrary, Treg cells, driven by Th17 cells, prevent the excessive differentiation of osteoclasts. While Th17 cells are a pathogenic subset of CD4+ T cells that produce IL-17 to promote osteoclastogenesis [104].
High expression of RANKL in T cells enhanced the survival of intestinal CD11c+ dendritic cells in mice lacking IL-2, resulting in bone loss [133]. Macrophages and dendritic cells, which exert pro-inflammatory effects, directly activate RANK signaling to promote osteoclastogenesis via secreting IL-1, IL-6 and TNF-α [31, 134], directly and macrophages and dendritic cells promote cytokines, such as IL-23 to induce osteoclast activation indirectly mediated by lymphocytes [135]. Macrophages and dendritic cells secrete IL-23 to inhibit osteoclast formation, blocking RANK dependent osteoclastogenesis through STAT1-dependent inhibition of c-Fos [136].
Osteoimmunology and cancer
Immune response determines the tumorigenesis and development of cancer. The bone niche with immune cells is a real “place of call” for tumor and cancer stem cells [137]. Both bone cancers and bone metastases are frequent complications of many cancers that cause bone complications, including fractures, bone pain and disability [138]. Bone is one of the most preferential metastatic target sites for certain cancers [139]. Stephen Paget proposed that bone is an exclusively favorable environment for tumor metastasis, namely the “seed and soil theory” [140].
The tumor microenvironment influences the behavior of cancer cells and immune cells, which further regulates cancer progression. In the tumor microenvironment, cancer cells and immune cells produce various cytokines or factors, such as PGE2, IL-6, IL-8, IL-11, and TNF-α, to induce the expression of RANKL in osteoblasts and osteocytes, resulting in osteoclastogenesis and bone resorption. This bone resorption subsequently releases calcium and growth factors such as TGF-β and IGFs from the degraded bone matrices, which further leads to tumor progression [141]. On the other hand, soluble RANKL promotes the homing of tumor cells to bone by interacting with RANK in tumor cells. Besides, chemokines derived from bone marrow stromal cells and bone marrow endothelial cells, such as C-X-C motif chemokine ligand 12, CX3C chemokine ligand 1, and C-C motif chemokine ligand 2, bind to their receptors in cancer cells and attract cancer cell migration to bone (Figure 4) [142, 143]. The above activities are referred to as a “vicious cycle”. NK cells promote the proliferation of cancer cells in the bone niche [144].
Crosstalk between bone and blood vessel
Cells expressing early cardiac markers are known to reside in the bone marrow and these progenitor cells not only act as markers of disease, but also contribute to myocardial healing in the setting of ischemic injury [145]. Recent work has demonstrated that regulatory hierarchies active in developing bone are also important in heart valve maturation [146]. The bone-vascular axis calcification paradox serves as a bridge between bone (osteoporosis) and vascular diseases (cardiovascular disease, CVD) [147]. CVD and cardiovascular mortality are associated with reduced bone mineral density and bone fractures. These two conditions may be sustained by similar mechanisms.
Bone to blood vessel
Osteoprotegerin (OPG) is a glycoprotein that belongs to the tumor necrosis factor superfamily. It is mainly expressed in osteoblasts, inhibits osteoclastogenesis, and inhibits bone loss by blocking the connection between RANKL [148] and its receptor RANK [149]. Clinical observations show that OPG participates in cardiovascular diseases. The concentration of plasma OPG in CVD patients is higher than that in the healthy volunteers [74]. These high concentrations of OPG are also associated with a greater range of atherosclerotic lesions in the coronary arteries and a higher risk of death [150]. Moreover, high concentrations of OPG are a predictor of a higher frequency of hospitalizations due to exacerbated ischemic heart failure with a reduced ejection fraction [74], and OPG concentrations in plasma were also related to patients with unstable angina and acute myocardial infarction [151]. There is increasing evidence that OPG not only is a marker of an unfavorable prognosis in CVDs, but also plays an important pathogenetic role in the development of cardiovascular diseases.
Blood vessel to bone
Osteogenesis and angiogenesis are intimately connected during bone formation and regeneration in the mammalian skeletal system [152]. Blood vessels provide bone tissues with the necessary nutrients, oxygen, growth factors, and hormones, and play an essential role in the regulation of bone formation [153]. Type H vessels induce bone formation by producing factors that stimulate the proliferation and differentiation of osteoprogenitors in the bone marrow [154].
Type I collagen, proteoglycan, osteopontin, osteonectin and OPG are found in bone and vascular matrix components and play important roles in bone formation and the development of atherosclerosis. Cytokines, such as IL-1, IL-6 and TNF-α also play roles in both CVDs and osteoporosis. Recently, Xie’s group uncovered an explanation for the calcification paradox. They found that the extracellular vesicles derived from aged bone matrix act as messengers and favor the adipogenesis of bone marrow stem cells rather than osteogenesis and augment calcification of vascular smooth muscle cells during bone resorption by transferring miR-483-5p and miR-2861 [155].
Crosstalk between bone and pancreas
Bone and pancreas also have tight connections. Dysfunction of the pancreas usually induces diabetes mellitus [156], which is a chronic metabolic derangement and leads to serious complications that may affect multiple organs, including bone. Moreover. diabetes mellitus is associated with an increased risk of fractures [157].
Bone to pancreas
Osteoglycin is a proteoglycan rich in leucine that derives from bone, cartilage and myocytes [158]. It enhances bone mineralization and formation by upregulating alkaline phosphatase and OCN in osteoblasts. Besides, the addition of active vitamin D upregulates osteoglycin expression in myoblasts. Osteoglycin is hypothesized to exert endocrine effects on bone and pancreas. Osteoglycin knockout mice revealed increased white adipose tissues, impaired glucose tolerance independent of diet consumption [159], higher femoral bone mineral contents and abnormally increased size of collagen fibrils [160].
OCN also connects the bone and pancreas (Figure 3). Clinical data show that the uncarboxylated OCN in serum negatively correlates with insulin resistance, obesity, diabetes, or markers of metabolic syndrome [161]. Moreover, weight loss causes a decrease in insulin resistance as well as an increase in OCN levels in obese children [162], and exercise-induced body fat reduction and improved insulin sensitivity were accompanied by increasing serum OCN and leptin levels [163]. OCN increases insulin sensitivity in liver, muscle, and adipose tissue by upregulating adiponectin expression in adipocytes [164]. Ocn KO mice reveal impaired glucose metabolism, including increased blood glucose, impaired glucose tolerance test and insulin tolerance test, and reduced β-cell mass and insulin content in the pancreas [68]. The uncarboxylated OCN enters the circulation and regulates the secretion and sensitivity of insulin [68]. In turn, the impaired glucose metabolism by dephosphorylating the insulin receptor (InsR) and inhibiting insulin signaling in osteoblasts leads to a reduction in bone resorption and uncarboxylated OCN [165].
In addition, osteocyte dysfunction also contributes to bone fragility in diabetes patients. Elevated serum sclerostin levels secreted by osteocytes were associated with prevalent vertebral fractures in type 2 diabetes mellitus patients [166]. In contrast, there are inconsistent voices about the function of osteoclasts in diabetes mellitus patients. Several studies revealed that bone resorption markers were higher in diabetes mellitus patients than those in non-diabetes donors [167]. While other studies showed opposite results that bone resorption markers were lower in diabetes mellitus patients [168, 169]. Thus, the roles and mechanisms of osteoclasts in diabetes need to be further clarified.
Pancreas to bone
Glycometabolism regulates bone metabolism and impairs bone microstructure. Diabetes (both type 1 and type 2 diabetes) or impaired glucose metabolism affects bone health, leading to decreasing bone formation, increasing bone marrow adiposity and the risk of fracture [170]. Osteoporosis is recognized as a diabetic complication [171]. It has been reported that the increased risk of fracture in diabetes is independent from the reduction of bone mineral density [172], suggesting that diabetes-induced fracture is mainly caused by deterioration of bone quality. Moreover, the higher prevalence of insulin resistance and worse β-cell function were associated with decreased bone turnover biomarkers, such as β-C-terminal telopeptide, N-terminal pro-peptide of type I collagen and OCN in dysglycemia patients [167].
Diabetes mellitus patients have higher levels of advanced glycation end products (AGEs) than non-diabetic subjects. The high levels of AGEs further affect bone metabolism [173]. Hyperglycemia increases the expression of the receptor for AGEs [174], which is expressed in osteoblasts and osteocytes [175, 176]. High glucose and AGEs inhibited the mineralization of osteoblasts [177], and AGEs inhibited osteoblastic differentiation or mineralization [178].
Insulin is a key growth hormone that plays an important role in glucose regulation by promoting glucose uptake in adipose tissue and muscle and coordinates the appropriate growth and development of the skeleton [179]. Pancreatectomy causes hypoinsulinaemia, hyperglycaemia and growth retardation, which further causes growth retardation of the axial and appendicular skeleton, and delayed ossification of limb bones [180]. Impairments in glucose and insulin metabolism affect bone quality directly by impacting on osteoblasts and osteoclasts, and affect bone remodeling activity indirectly via regulating bone vasculature, which is critical for bone growth, remodeling and injury healing. Ultimately, the indirect and direct effects lead to an increased risk of fractures [181]. Osteoblasts express abundant InsR [182] to promote cell proliferation [183] and collagen synthesis [184]. Specific knockout of InsR in osteoblasts decreases the number of osteoblasts and bone formation by suppressing the Runx2 inhibitor Twist2 [185]. In addition, knockdown of InsR by shRNA effectively suppressed osteoclast differentiation via the ERK1/2 signaling pathway [186]. InsR signal decreases the ability of the forkhead box O1 transcription factor to activate the OPG promoter, resulting in reducing osteoclastogenesis [187].
Crosstalk between bone and kidney
Bone and kidney connection. Chronic kidney disease-mineral and bone disorder (CKD-MBD) is a complex syndrome of renal osteodystrophy, mineral disturbances and cardiovascular disease that has become a global health crisis with very limited therapeutic options. Sclerostin, DKK1, FGF23 and OCN have recently been presented as new therapeutic targets for CKD-MBD [188].
Bone to kidney
Sclerostin and DKK1 are two powerful inhibitors of the canonical Wnt pathway that play a role in CKD-MBD [189]. The increased circulating levels of both sclerostin and DKK1 impaired kidney function, even inducing end stage renal disease as a result [1, 190]. FGF23 has become one of the most important osteocyte-secreted endocrine factors [191]. It is secreted by osteoblasts and osteocytes and is the first hormone found in bone tissue that regulates the systemic phosphate and vitamin D [192]. Sclerostin stimulates FGF23 synthesis and exerts indirect effects on mineral metabolism [193]. The high levels of serum sclerostin observed in CKD might promote resistance to parathyroid hormone (PTH), leading to adynamic bone disease. Furthermore, the levels of sclerostin are highest in those with vascular calcification and kidney failure compared to those with normal renal function and calcification [194]. Bone biopsy revealed a negative correlation between serum sclerostin and PTH in patients on dialysis [195]. DKK1 is also involved in FGF23/α-Klotho-mediated bone loss in CKD [196]. A significant decrease in DKK1 was associated with a decline in renal function [197].
Kidney to bone
FGF23 inhibits renal tubular reabsorption of phosphate by reducing the production and secretion of PTH and is a completely new player in CKD-MBD [198]. Circulating FGF23 represents the biochemical substrate of the inter-organ communication between bone and kidney and regulates P handling and vitamin D synthesis in renal tubular cells by binding to FGF receptors, which requires the type I transmembrane protein α-Klotho, a co-receptor. α-Klotho is mainly produced in the kidney [199], parathyroid gland, and choroid plexus [199]. FGF23 levels increase dramatically as an extreme bone response to the burden of P load and altered catabolism in the end-stage renal disease [200]. Intermittent administration of PTH in the early CKD stage to avert the increase in serum phosphorus and FGF-23 may be able to prevent CKD–MBD development.
Crosstalk between bone and liver
Liver is the site of glucose storage, which is initiated by insulin [201]. There is increasing evidence for the bone-liver axis [202]. Interaction between liver and bone is also bidirectional: metabolism of liver may affect and be affected by bone metabolism. Nonalcoholic fatty liver disease (NAFLD) is a global public health problem characterized by inflammation and/or fibrosis [203], ranging from nonalcoholic simple steatosis to nonalcoholic steatohepatitis [204]. Moreover, NAFLD is closely linked to osteoporosis [205].
Bone to liver
Hepatic osteodystrophy is another metabolic bone disease marked by bone loss and is associated with chronic liver disease [206]. Ehnert’s group showed that hepatic expression of the phosphatase PP2Acα is upregulated during hepatic osteodystrophy, leading to the downregulation of expression of the hepatokine, which significantly exacerbates the bone loss phenotype of hepatic osteodystrophy. Additionally, lecithin-cholesterol acyltransferase improves liver function and relieves liver fibrosis hepatic osteodystrophy by promoting reversal of cholesterol transport from bone to the liver [207]. This study demonstrates that defects in a liver-bone axis can be effective treatment targets to ameliorate HOD progression.
Sclerostin and DKK1 inhibit osteoblast differentiation and bone formation via suppressing the Wnt/β-catenin signaling pathway [208, 209]. Moreover, sclerostin levels in serum were lower in nonalcoholic steatohepatitis patients than in healthy donors, and DKK1 levels were independently associated with nonalcoholic steatohepatitis in NAFLD patients [210]. Circulating OCN is negatively associated with NAFLD [211] and uncarboxylated OCN ameliorates histological hepatic steatosis via activating the insulin signaling pathway [212]. It was further shown that the OCN receptor GPRC6A is highly expressed in liver hepatocytes (Figure 3) [213], suggesting that OCN might directly regulate liver lipid homeostasis.
Liver to bone
Growth hormone (GH) is secreted by the pituitary gland and acts upon the liver. GH binds to the GH receptor and activates the downstream JAK2/STAT5 signaling [214]. Furthermore, activated STAT5 targets the IGF-1 gene in the nucleus of the liver and secrets IGF-1 to ultimately contribute to bone remodeling by regulating osteoblasts and osteoclasts [215]. Besides, there is a positive correlation between bone mineral density and circulating levels of IGF-1 [216]. It has been reported that deficiency of IGF-1 in the liver results in a 25% reduction in bone volume [217], and decreased levels of circulating IGF-1 are strongly associated with a 40% increase in fracture risk [145].
Crosstalk between bone and gonad
The sex steroid hormones testosterone and estrogen secreted by reproductive organs are essential determinants not only of reproductive functions, but also for bone growth and bone balance. This theory is best exemplified by the fact that osteoporosis always occurs in postmenopausal women. The crosstalk between bone and gonad owe to OCN, which promotes testosterone biosynthesis, principally. Ocn KO mice exhibit an elevated adiposity, impaired glucose tolerance and a low survival rate during embryonic periods.
Bone to gonad
In the last decade, the disclosure of systemic roles of the undercarboxylated form of OCN contributed to switching the concept of bone from a merely structural apparatus to a fully endocrine organ participated in the regulation of systemic functions. Male Ocn KO mice were rather poor breeders [65]. Specifically, Ocn KO mice showed a decrease in testes, epididymides and seminal vesicle weights and a 50% decrease in sperm count [218]. Similarly, OCN secreted by bone cells binds to GPRC6A expressed in Leydig cells of the testes (Figure 2). Furthermore, OCN promotes testosterone production by the testis in a cAMP response element binding-dependent manner.
Gonad to bone
It is easy to understand how sex steroid hormones influence bone metabolism, because menopause causes bone loss. Sex steroids play a crucial role during the bone growth spurts of puberty and for the maintenance of bone mass. During puberty, gender differences in bone growth become apparent with men reaching higher peak bone mass, which is attributed to a stimulatory androgen effect on periosteal bone formation in men, but estrogen shows an inhibitory role in bone formation in women. Testosterone and estrogens also participate in the maintenance of bone mass integrity during adulthood in both female and male. Then, the decrease in testosterone or estrogen levels with age or in gonadal dysfunction leads to a decrease in bone mass and increases the risk of osteoporosis. At the cellular level, estrogen decrease the generation, lifespan, and functional activity of osteoclasts via reducing the production of IL-1, IL-6, TNF-β, RANKL and M-CSF [219] and show an opposite effect on osteoblasts via the release of reactive oxygen species [220, 221].
Conclusions
The focus of modern biology is no longer on an isolated single system, but on an integrated multi-system. Bone is a conserved and ancient organ that plays important roles in supporting the body and protecting the viscera. Traditionally, the understanding of bone is simple, mechanical and rigid. In recent years, the functions of bone with respect to other organs as an endocrine organ have received increasing attention. Bone cannot be completely removed, so its function cannot be utterly studied like other organs, such as the heart, liver or kidney. With the development of gene technology, especially bone specific gene knockout technology, the function of bone has been discovered gradually. The crosstalk between bone and other organs opens a brand-new door and provides a new therapeutic target for the treatment of the systemic diseases.
Supplementary Material
Supplementary Material
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/mr-2022-0018).
Footnotes
Research funding: This work was supported by the National key research and development program (2020YFC2009004, 2021YFC2501700), National Natural Science Foundation of China (81874010) and PKU-Baidu Fund (2020BD014).
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
References
- 1.Robling AG, Bonewald LF. The osteocyte: new insights. Annu Rev Physiol. 2020;82:485–506. doi: 10.1146/annurev-physiol-021119-034332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asada N, Sato M, Katayama Y. Communication of bone cells with hematopoiesis, immunity and energy metabolism. BoneKEy Rep. 2015;4:748. doi: 10.1038/bonekey.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karsenty G, Olson EN. Bone and muscle endocrine functions: unexpected paradigms of inter-organ communication. Cell. 2016;164:1248–56. doi: 10.1016/j.cell.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kozlowska U, Krawczenko A, Futoma K, Jurek T, Rorat M, Patrzalek D, et al. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues. World J Stem Cell. 2019;11:347–74. doi: 10.4252/wjsc.v11.i6.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirschfeld HP, Kinsella R, Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int. 2017;28:2781–90. doi: 10.1007/s00198-017-4151-8. [DOI] [PubMed] [Google Scholar]
- 6.Clynes MA, Gregson CL, Bruyere O, Cooper C, Dennison EM. Osteosarcopenia: where osteoporosis and sarcopenia collide. Rheumatology. 2021;60:529–37. doi: 10.1093/rheumatology/keaa755. [DOI] [PubMed] [Google Scholar]
- 7.Gomarasca M, Banfi G, Lombardi G. Myokines: the endocrine coupling of skeletal muscle and bone. Adv Clin Chem. 2020;94:155–218. doi: 10.1016/bs.acc.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Li G, Zhang L, Wang D, Aiqudsy L, Jiang JX, Xu H, et al. Muscle-bone crosstalk and potential therapies for sarco-osteoporosis. J Cell Biochem. 2019;120:14262–73. doi: 10.1002/jcb.28946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mera P, Laue K, Ferron M, Confavreux C, Wei J, Galan-Diez M, et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metabol. 2016;23:1078–92. doi: 10.1016/j.cmet.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mera P, Laue K, Wei J, Berger JM, Karsenty G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol Metabol. 2016;5:1042–7. doi: 10.1016/j.molmet.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin X, Brennan-Speranza TC, Levinger I, Yeap BB. Undercarboxylated osteocalcin: experimental and human evidence for a role in glucose homeostasis and muscle regulation of insulin sensitivity. Nutrients. 2018;10:847. doi: 10.3390/nu10070847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–65. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 13.Huang J, Romero-Suarez S, Lara N, Mo C, Kaja S, Brotto L, et al. Crosstalk between mlo-y4 osteocytes and c2c12 muscle cells is mediated by the wnt/beta-catenin pathway. JBMR Plus. 2017;1:86–100. doi: 10.1002/jbm4.10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpenter KA, Ross RD. Sclerostin antibody treatment increases bone mass and normalizes circulating phosphate levels in growing hyp mice. J Bone Miner Res. 2020;35:596–607. doi: 10.1002/jbmr.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sebastian A, Loots GG. Genetics of sost/sost in sclerosteosis and van buchem disease animal models. Metabolism. 2018;80:38–47. doi: 10.1016/j.metabol.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of sost/sclerostin. J Biol Chem. 2008;283:5866–75. doi: 10.1074/jbc.m705092200. [DOI] [PubMed] [Google Scholar]
- 17.Spatz JM, Fields EE, Yu EW, Divieti PP, Bouxsein ML, Sibonga JD, et al. Serum sclerostin increases in healthy adult men during bed rest. J Clin Endocrinol Metab. 2012;97:E1736–40. doi: 10.1210/jc.2012-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho A, Palla AR, Blake MR, Yucel ND, Wang YX, Magnusson K, et al. Prostaglandin e2 is essential for efficacious skeletal muscle stem-cell function, augmenting regeneration and strength. Proc Natl Acad Sci U S A. 2017;114:6675–84. doi: 10.1073/pnas.1705420114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamel MA, Picconi JL, Lara-Castillo N, Johnson ML. Activation of beta-catenin signaling in mlo-y4 osteocytic cells versus 2t3 osteoblastic cells by fluid flow shear stress and pge2: implications for the study of mechanosensation in bone. Bone. 2010;47:872–81. doi: 10.1016/j.bone.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mo C, Zhao R, Vallejo J, Igwe O, Bonewald L, Wetmore L, et al. Prostaglandin e2 promotes proliferation of skeletal muscle myoblasts via ep4 receptor activation. Cell Cycle. 2015;14:1507–16. doi: 10.1080/15384101.2015.1026520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palla AR, Ravichandran M, Wang YX, Alexandrova L, Yang AV, Kraft P, et al. Inhibition of prostaglandin-degrading enzyme 15-pgdh rejuvenates aged muscle mass and strength. Science. 2021;371:eabc8059. doi: 10.1126/science.abc8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendias CL, Gumucio JP, Davis ME, Bromley CW, Davis CS, Brooks SV. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle Nerve. 2012;45:55–9. doi: 10.1002/mus.22232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waning DL, Mohammad KS, Reiken S, Xie W, Andersson DC, John S, et al. Excess tgf-beta mediates muscle weakness associated with bone metastases in mice. Nat Med. 2015;21:1262–71. doi: 10.1038/nm.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mcdonald MM, Khoo WH, Ng PY, Xiao Y, Zamerli J, Thatcher P, et al. Osteoclasts recycle via osteomorphs during rankl-stimulated bone resorption. Cell. 2021;184:1940. doi: 10.1016/j.cell.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dufresne SS, Dumont NA, Boulanger-Piette A, Fajardo VA, Gamu D, Kake-Guena SA, et al. Muscle rank is a key regulator of ca2+ storage, serca activity, and function of fast-twitch skeletal muscles. Am J Physiol Cell Physiol. 2016;310:C663–72. doi: 10.1152/ajpcell.00285.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamoudi D, Marcadet L, Piette BA, Yagita H, Bouredji Z, Argaw A, et al. An anti-rankl treatment reduces muscle inflammation and dysfunction and strengthens bone in dystrophic mice. Hum Mol Genet. 2019;28:3101–12. doi: 10.1093/hmg/ddz124. [DOI] [PubMed] [Google Scholar]
- 27.Bonnet N, Bourgoin L, Biver E, Douni E, Ferrari S. Rankl inhibition improves muscle strength and insulin sensitivity and restores bone mass. J Clin Invest. 2020;130:3329. doi: 10.1172/jci138278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chowdhury S, Schulz L, Palmisano B, Singh P, Berger JM, Yadav VK, et al. Muscle-derived interleukin 6 increases exercise capacity by signaling in osteoblasts. J Clin Invest. 2020;130:2888–902. doi: 10.1172/jci133572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lombardi G, Sanchis-Gomar F, Perego S, Sansoni V, Banfi G. Implications of exercise-induced adipo-myokines in bone metabolism. Endocrine. 2016;54:284–305. doi: 10.1007/s12020-015-0834-0. [DOI] [PubMed] [Google Scholar]
- 30.Lombardi G, Perego S, Luzi L, Banfi G. A four-season molecule: osteocalcin. Updates in its physiological roles. Endocrine. 2015;48:394–404. doi: 10.1007/s12020-014-0401-0. [DOI] [PubMed] [Google Scholar]
- 31.Yokota K, Sato K, Miyazaki T, Aizaki Y, Tanaka S, Sekikawa M, et al. Characterization and function of tumor necrosis factor and interleukin-6-induced osteoclasts in rheumatoid arthritis. Arthritis Rheumatol. 2021;73:1145–54. doi: 10.1002/art.41666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juffer P, Jaspers RT, Klein-Nulend J, Bakker AD. Mechanically loaded myotubes affect osteoclast formation. Calcif Tissue Int. 2014;94:319–26. doi: 10.1007/s00223-013-9813-8. [DOI] [PubMed] [Google Scholar]
- 33.Tagliaferri C, Wittrant Y, Davicco MJ, Walrand S, Coxam V. Muscle and bone, two interconnected tissues. Ageing Res Rev. 2015;21:55–70. doi: 10.1016/j.arr.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Sharma M, Mcfarlane C, Kambadur R, Kukreti H, Bonala S, Srinivasan S. Myostatin: expanding horizons. IUBMB Life. 2015;67:589–600. doi: 10.1002/iub.1392. [DOI] [PubMed] [Google Scholar]
- 35.Allen DL, Cleary AS, Speaker KJ, Lindsay SF, Uyenishi J, Reed JM, et al. Myostatin, activin receptor iib, and follistatin-like-3 gene expression are altered in adipose tissue and skeletal muscle of obese mice. Am J Physiol Endocrinol Metab. 2008;294:E918–27. doi: 10.1152/ajpendo.00798.2007. [DOI] [PubMed] [Google Scholar]
- 36.Bonewald L. Use it or lose it to age: a review of bone and muscle communication. Bone. 2019;120:212–8. doi: 10.1016/j.bone.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamrick MW. The skeletal muscle secretome: an emerging player in muscle-bone crosstalk. BoneKEy Rep. 2012;1:60. doi: 10.1038/bonekey.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bialek P, Parkington J, Li X, Gavin D, Wallace C, Zhang J, et al. A myostatin and activin decoy receptor enhances bone formation in mice. Bone. 2014;60:162–71. doi: 10.1016/j.bone.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Omosule CL, Gremminger VL, Aguillard AM, Jeong Y, Harrelson EN, Miloscio L, et al. Impact of genetic and pharmacologic inhibition of myostatin in a murine model of osteogenesis imperfecta. J Bone Miner Res. 2021;36:739–56. doi: 10.1002/jbmr.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin Y, Peng Y, Zhao W, Pan J, Ksiezak-Reding H, Cardozo C, et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microrna-218: a novel mechanism in muscle-bone communication. J Biol Chem. 2017;292:11021–33. doi: 10.1074/jbc.m116.770941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyu H, Xiao Y, Guo Q, Huang Y, Luo X. The role of bone-derived exosomes in regulating skeletal metabolism and extraosseous diseases. Front Cell Dev Biol. 2020;8:89. doi: 10.3389/fcell.2020.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dankbar B, Fennen M, Brunert D, Hayer S, Frank S, Wehmeyer C, et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat Med. 2015;21:1085–90. doi: 10.1038/nm.3917. [DOI] [PubMed] [Google Scholar]
- 43.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A pgc1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buccoliero C, Oranger A, Colaianni G, Pignataro P, Zerlotin R, Lovero R, et al. The effect of irisin on bone cells in vivo and in vitro. Biochem Soc Trans. 2021;49:477–84. doi: 10.1042/bst20200978. [DOI] [PubMed] [Google Scholar]
- 45.Ma Y, Qiao X, Zeng R, Cheng R, Zhang J, Luo Y, et al. Irisin promotes proliferation but inhibits differentiation in osteoclast precursor cells. Faseb J. 2018;32:fj201700983RR. doi: 10.1096/fj.201700983RR. [DOI] [PubMed] [Google Scholar]
- 46.Xu L, Shen L, Yu X, Li P, Wang Q, Li C. Effects of irisin on osteoblast apoptosis and osteoporosis in postmenopausal osteoporosis rats through upregulating nrf2 and inhibiting nlrp3 inflammasome. Exp Ther Med. 2020;19:1084–90. doi: 10.3892/etm.2019.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim H, Wrann CD, Jedrychowski M, Vidoni S, Kitase Y, Nagano K, et al. Irisin mediates effects on bone and fat via alphav integrin receptors. Cell. 2018;175:1756–68. doi: 10.1016/j.cell.2018.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishizawa H, Matsuda M, Yamada Y, Kawai K, Suzuki E, Makishima M, et al. Musclin, a novel skeletal muscle-derived secretory factor. J Biol Chem. 2004;279:19391–5. doi: 10.1074/jbc.c400066200. [DOI] [PubMed] [Google Scholar]
- 49.Moffatt P, Thomas GP. Osteocrin--beyond just another bone protein? Cell Mol Life Sci. 2009;66:1135–9. doi: 10.1007/s00018-009-8716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yasui A, Nishizawa H, Okuno Y, Morita K, Kobayashi H, Kawai K, et al. Foxo1 represses expression of musclin, a skeletal muscle-derived secretory factor. Biochem Biophys Res Commun. 2007;364:358–65. doi: 10.1016/j.bbrc.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 51.Hamrick MW. A role for myokines in muscle-bone interactions. Exerc Sport Sci Rev. 2011;39:43–7. doi: 10.1097/jes.0b013e318201f601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamrick MW, Mcneil PL, Patterson SL. Role of muscle-derived growth factors in bone formation. J Musculoskelet Neuronal Interact. 2010;10:64–70. [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y, Nishida S, Elalieh HZ, Long RK, Halloran BP, Bikle DD. Role of igf-i signaling in regulating osteoclastogenesis. J Bone Miner Res. 2006;21:1350–8. doi: 10.1359/jbmr.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheng MH, Zhou XD, Bonewald LF, Baylink DJ, Lau KH. Disruption of the insulin-like growth factor-1 gene in osteocytes impairs developmental bone growth in mice. Bone. 2013;52:133–44. doi: 10.1016/j.bone.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 55.Locatelli V, Bianchi VE. Effect of gh/igf-1 on bone metabolism and osteoporsosis. Int J Endocrinol. 2014;2014:235060. doi: 10.1155/2014/235060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Juffer P, Jaspers RT, Lips P, Bakker AD, Klein-Nulend J. Expression of muscle anabolic and metabolic factors in mechanically loaded mlo-y4 osteocytes. Am J Physiol Endocrinol Metab. 2012;302:E389–95. doi: 10.1152/ajpendo.00320.2011. [DOI] [PubMed] [Google Scholar]
- 57.Zhu Z, Huang P, Chong Y, George SK, Wen B, Han N, et al. Nucleus pulposus cells derived igf-1 and mcp-1 enhance osteoclastogenesis and vertebrae disruption in lumbar disc herniation. Int J Clin Exp Pathol. 2014;7:8520–31. [PMC free article] [PubMed] [Google Scholar]
- 58.Benington L, Rajan G, Locher C, Lim LY. Fibroblast growth factor 2-a review of stabilisation approaches for clinical applications. Pharmaceutics. 2020;12:508. doi: 10.3390/pharmaceutics12060508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adhikary S, Choudhary D, Tripathi AK, Karvande A, Ahmad N, Kothari P, et al. Fgf-2 targets sclerostin in bone and myostatin in skeletal muscle to mitigate the deleterious effects of glucocorticoid on musculoskeletal degradation. Life Sci. 2019;229:261–76. doi: 10.1016/j.lfs.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 60.Carda S, Cisari C, Invernizzi M, Bevilacqua M. Osteoporosis after stroke: a review of the causes and potential treatments. Cerebrovasc Dis. 2009;28:191–200. doi: 10.1159/000226578. [DOI] [PubMed] [Google Scholar]
- 61.Kwon MJ, Kim JH, Kim JH, Cho SJ, Nam ES, Choi HG. The occurrence of alzheimer’s disease and Parkinson’s disease in individuals with osteoporosis: a longitudinal follow-up study using a national health screening database in korea. Front Aging Neurosci. 2021;13:786337. doi: 10.3389/fnagi.2021.786337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–52. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 63.Diegel CR, Hann S, Ayturk UM, Hu J, Lim KE, Droscha CJ, et al. An osteocalcin-deficient mouse strain without endocrine abnormalities. PLoS Genet. 2020;16:e1008361. doi: 10.1371/journal.pgen.1008361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Obri A, Khrimian L, Karsenty G, Oury F. Osteocalcin in the brain: from embryonic development to age-related decline in cognition. Nat Rev Endocrinol. 2018;14:174–82. doi: 10.1038/nrendo.2017.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144:796–809. doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix gla protein: vitamin k-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 67.Mizokami A, Kawakubo-Yasukochi T, Hirata M. Osteocalcin and its endocrine functions. Biochem Pharmacol. 2017;132:1–8. doi: 10.1016/j.bcp.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 68.Ferron M, Wei J, Yoshizawa T, Del FA, Depinho RA, Teti A, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pi M, Kapoor K, Ye R, Nishimoto SK, Smith JC, Baudry J, et al. Evidence for osteocalcin binding and activation of gprc6a in beta-cells. Endocrinology. 2016;157:1866–80. doi: 10.1210/en.2015-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khrimian L, Obri A, Ramos-Brossier M, Rousseaud A, Moriceau S, Nicot AS, et al. Gpr158 mediates osteocalcin’s regulation of cognition. J Exp Med. 2017;214:2859–73. doi: 10.1084/jem.20171320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kosmidis S, Polyzos A, Harvey L, Youssef M, Denny CA, Dranovsky A, et al. Rbap48 protein is a critical component of gpr158/ocn signaling and ameliorates age-related memory loss. Cell Rep. 2018;25:959–73. doi: 10.1016/j.celrep.2018.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Braverman ER, Chen TJ, Chen AL, Arcuri V, Kerner MM, Bajaj A, et al. Age-related increases in parathyroid hormone may be antecedent to both osteoporosis and dementia. BMC Endocr Disord. 2009;9:21. doi: 10.1186/1472-6823-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oury F, Khrimian L, Denny CA, Gardin A, Chamouni A, Goeden N, et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013;155:228–41. doi: 10.1016/j.cell.2013.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Montagnana M, Lippi G, Danese E, Guidi GC. The role of osteoprotegerin in cardiovascular disease. Ann Med. 2013;45:254–64. doi: 10.3109/07853890.2012.727019. [DOI] [PubMed] [Google Scholar]
- 75.Mosialou I, Shikhel S, Liu JM, Maurizi A, Luo N, He Z, et al. Mc4r-dependent suppression of appetite by bone-derived lipocalin 2. Nature. 2017;543:385–90. doi: 10.1038/nature21697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koide M, Kobayashi Y. Regulatory mechanisms of sclerostin expression during bone remodeling. J Bone Miner Metab. 2019;37:9–17. doi: 10.1007/s00774-018-0971-7. [DOI] [PubMed] [Google Scholar]
- 77.Jia L, Pina-Crespo J, Li Y. Restoring wnt/beta-catenin signaling is a promising therapeutic strategy for alzheimer’s disease. Mol Brain. 2019;12:104. doi: 10.1186/s13041-019-0525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bai B, Wang X, Li Y, Chen PC, Yu K, Dey KK, et al. Deep multilayer brain proteomics identifies molecular networks in alzheimer’s disease progression. Neuron. 2020;105:975–91. doi: 10.1016/j.neuron.2019.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuan J, Meloni BP, Shi T, Bonser A, Papadimitriou JM, Mastaglia FL, et al. The potential influence of bone-derived modulators on the progression of alzheimer’s disease. J Alzheimers Dis. 2019;69:59–70. doi: 10.3233/jad-181249. [DOI] [PubMed] [Google Scholar]
- 80.Han KH, Arlian BM, Macauley MS, Paulson JC, Lerner RA. Migration-based selections of antibodies that convert bone marrow into trafficking microglia-like cells that reduce brain amyloid beta. Proc Natl Acad Sci U S A. 2018;115:E372–81. doi: 10.1073/pnas.1719259115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elefteriou F. Impact of the autonomic nervous system on the skeleton. Physiol Rev. 2018;98:1083–112. doi: 10.1152/physrev.00014.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao S, Definis JH, Hou S. Alterations of dopamine-related transcripts in a11 diencephalospinal pathways after spinal cord injury. Neural Plast. 2021;2021:8838932. doi: 10.1155/2021/8838932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim SM, Taneja C, Perez-Pena H, Ryu V, Gumerova A, Li W, et al. Repurposing erectile dysfunction drugs tadalafil and vardenafil to increase bone mass. Proc Natl Acad Sci U S A. 2020;117:14386–94. doi: 10.1073/pnas.2000950117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moraes RM, Elefteriou F, Anbinder AL. Response of the periodontal tissues to beta-adrenergic stimulation. Life Sci. 2021;281:119776. doi: 10.1016/j.lfs.2021.119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Speidel J, Mattucci S, Liu J, Kwon BK, Tetzlaff W, Oxland TR. Effect of velocity and duration of residual compression in a rat dislocation spinal cord injury model. J Neurotrauma. 2020;37:1140–8. doi: 10.1089/neu.2019.6747. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 87.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481:314–20. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jin LY, Guo C, Xu S, Liu HY, Li XF. The role of estrogen receptor alpha in response to longitudinal bone growth in ob/ob mice. Front Endocrinol. 2021;12:749449. doi: 10.3389/fendo.2021.749449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Philbrick KA, Wong CP, Branscum AJ, Turner RT, Iwaniec UT. Leptin stimulates bone formation in ob/ob mice at doses having minimal impact on energy metabolism. J Endocrinol. 2017;232:461–74. doi: 10.1530/joe-16-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kumar KK, Tung S, Iqbal J. Bone loss in anorexia nervosa: leptin, serotonin, and the sympathetic nervous system. Ann N Y Acad Sci. 2010;1211:51–65. doi: 10.1111/j.1749-6632.2010.05810.x. [DOI] [PubMed] [Google Scholar]
- 91.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of socs-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–25. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 92.Wang QF, Sun Z, Zheng FR, Zhang GW, Liu Z. Association of adrenergic receptor alpha2a (alpha2a-ar) gene rs1800544 polymorphism with bone mineral density and bone turnover markers in an elderly Chinese population. Med Sci Monit. 2018;24:5102–9. doi: 10.12659/msm.908376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bellinger DL, Wood C, Wergedal JE, Lorton D. Driving beta2- while suppressing alpha-adrenergic receptor activity suppresses joint pathology in inflammatory arthritis. Front Immunol. 2021;12:628065. doi: 10.3389/fimmu.2021.628065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Z, Liu Y, Zhang J, Lin M, Xiao C, Bai H, et al. Mechanical loading alleviated the inhibition of beta2-adrenergic receptor agonist terbutaline on bone regeneration. Faseb J. 2021;35:e22033. doi: 10.1096/fj.202101045RR. [DOI] [PubMed] [Google Scholar]
- 95.Madel MB, Elefteriou F. Mechanisms supporting the use of beta-blockers for the management of breast cancer bone metastasis. Cancers. 2021;13:2887–904. doi: 10.3390/cancers13122887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, et al. Leptin regulation of bone resorption by the sympathetic nervous system and cart. Nature. 2005;434:514–20. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 97.Bajayo A, Bar A, Denes A, Bachar M, Kram V, Attar-Namdar M, et al. Skeletal parasympathetic innervation communicates central il-1 signals regulating bone mass accrual. Proc Natl Acad Sci U S A. 2012;109:15455–60. doi: 10.1073/pnas.1206061109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. Osteoprotection by semaphorin 3a. Nature. 2012;485:69–74. doi: 10.1038/nature11000. [DOI] [PubMed] [Google Scholar]
- 99.Wright DE, White FA, Gerfen RW, Silos-Santiago I, Snider WD. The guidance molecule semaphorin iii is expressed in regions of spinal cord and periphery avoided by growing sensory axons. J Comp Neurol. 1995;361:321–33. doi: 10.1002/cne.903610209. [DOI] [PubMed] [Google Scholar]
- 100.Horton JE, Raisz LG, Simmons HA, Oppenheim JJ, Mergenhagen SE. Bone resorbing activity in supernatant fluid from cultured human peripheral blood leukocytes. Science. 1972;177:793–5. doi: 10.1126/science.177.4051.793. [DOI] [PubMed] [Google Scholar]
- 101.Arron JR, Choi Y. Bone versus immune system. Nature. 2000;408:535–6. doi: 10.1038/35046196. [DOI] [PubMed] [Google Scholar]
- 102.Okamoto K, Nakashima T, Shinohara M, Negishi-Koga T, Komatsu N, Terashima A, et al. Osteoimmunology: the conceptual framework unifying the immune and skeletal systems. Physiol Rev. 2017;97:1295–349. doi: 10.1152/physrev.00036.2016. [DOI] [PubMed] [Google Scholar]
- 103.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 104.Fischer L, Herkner C, Kitte R, Dohnke S, Riewaldt J, Kretschmer K, et al. Foxp3(+) regulatory t cells in bone and hematopoietic homeostasis. Front Endocrinol. 2019;10:578. doi: 10.3389/fendo.2019.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matejuk A. Skin immunity. Arch Immunol Ther Exp. 2018;66:45–54. doi: 10.1007/s00005-017-0477-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wagner DO, Aspenberg P. Where did bone come from? Acta Orthop. 2011;82:393–8. doi: 10.3109/17453674.2011.588861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Holick MF, Chen TC, Lu Z, Sauter E. Vitamin d and skin physiology: a d-lightful story. J Bone Miner Res. 2007;22:V28–33. doi: 10.1359/jbmr.07s211. Suppl 2. [DOI] [PubMed] [Google Scholar]
- 108.Mueller CG, Voisin B. Of skin and bone: did langerhans cells and osteoclasts evolve from a common ancestor? J Anat. 2019;235:412–7. doi: 10.1111/joa.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–34. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ono T, Hayashi M, Sasaki F, Nakashima T. Rankl biology: bone metabolism, the immune system, and beyond. Inflamm Regen. 2020;40:2. doi: 10.1186/s41232-019-0111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Walsh MC, Choi Y. Regulation of t cell-associated tissues and t cell activation by rankl-rank-opg. J Bone Miner Metab. 2021;39:54–63. doi: 10.1007/s00774-020-01178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mueller CG, Hess E. Emerging functions of rankl in lymphoid tissues. Front Immunol. 2012;3:261. doi: 10.3389/fimmu.2012.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gomez-Aleza C, Nguyen B, Yoldi G, Ciscar M, Barranco A, Hernandez-Jimenez E, et al. Inhibition of rank signaling in breast cancer induces an anti-tumor immune response orchestrated by cd8+ t cells. Nat Commun. 2020;11:6335. doi: 10.1038/s41467-020-20138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ilchovska DD, Barrow DM. An overview of the nf-kb mechanism of pathophysiology in rheumatoid arthritis, investigation of the nf-kb ligand rankl and related nutritional interventions. Autoimmun Rev. 2021;20:102741. doi: 10.1016/j.autrev.2020.102741. [DOI] [PubMed] [Google Scholar]
- 115.Guerrini MM, Okamoto K, Komatsu N, Sawa S, Danks L, Penninger JM, et al. Inhibition of the tnf family cytokine rankl prevents autoimmune inflammation in the central nervous system. Immunity. 2015;43:1174–85. doi: 10.1016/j.immuni.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 116.Khafipour A, Eissa N, Munyaka PM, Rabbi MF, Kapoor K, Kermarrec L, et al. Denosumab regulates gut microbiota composition and cytokines in dinitrobenzene sulfonic acid (dnbs)-experimental colitis. Front Microbiol. 2020;11:1405. doi: 10.3389/fmicb.2020.01405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Onder L, Morbe U, Pikor N, Novkovic M, Cheng HW, Hehlgans T, et al. Lymphatic endothelial cells control initiation of lymph node organogenesis. Immunity. 2017;47:80–92. doi: 10.1016/j.immuni.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 118.Green EA, Choi Y, Flavell RA. Pancreatic lymph node-derived cd4(+)cd25(+) treg cells: highly potent regulators of diabetes that require trance-rank signals. Immunity. 2002;16:183–91. doi: 10.1016/s1074-7613(02)00279-0. [DOI] [PubMed] [Google Scholar]
- 119.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. Opgl is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 120.Perlot T, Penninger JM. Development and function of murine b cells lacking rank. J Immunol. 2012;188:1201–5. doi: 10.4049/jimmunol.1102063. [DOI] [PubMed] [Google Scholar]
- 121.Yu VW, Saez B, Cook C, Lotinun S, Pardo-Saganta A, Wang YH, et al. Specific bone cells produce dll4 to generate thymus-seeding progenitors from bone marrow. J Exp Med. 2015;212:759–74. doi: 10.1084/jem.20141843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, et al. Cxcl12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–30. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Giuliani N, Colla S, Morandi F, Lazzaretti M, Sala R, Bonomini S, et al. Myeloma cells block runx2/cbfa1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood. 2005;106:2472–83. doi: 10.1182/blood-2004-12-4986. [DOI] [PubMed] [Google Scholar]
- 124.Faienza MF, Ventura A, Marzano F, Cavallo L. Postmenopausal osteoporosis: the role of immune system cells. Clin Dev Immunol. 2013;2013:575936. doi: 10.1155/2013/575936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, et al. Estrogen deficiency induces bone loss by enhancing t-cell production of tnf-alpha. J Clin Invest. 2000;106:1229–37. doi: 10.1172/jci11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lehmann J, Thiele S, Baschant U, Rachner TD, Niehrs C, Hofbauer LC, et al. Mice lacking dkk1 in t cells exhibit high bone mass and are protected from estrogen-deficiency-induced bone loss. iScience. 2021;24:102224. doi: 10.1016/j.isci.2021.102224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tsukasaki M, Takayanagi H. Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat Rev Immunol. 2019;19:626–42. doi: 10.1038/s41577-019-0178-8. [DOI] [PubMed] [Google Scholar]
- 128.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper t cell subset that links t cell activation and bone destruction. J Exp Med. 2006;203:2673–82. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, et al. Cutting edge: estrogen drives expansion of the cd4+cd25+ regulatory t cell compartment. J Immunol. 2004;173:2227–30. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- 130.Yuan FL, Li X, Lu WG, Xu RS, Zhao YQ, Li CW, et al. Regulatory t cells as a potent target for controlling bone loss. Biochem Biophys Res Commun. 2010;402:173–6. doi: 10.1016/j.bbrc.2010.09.120. [DOI] [PubMed] [Google Scholar]
- 131.Tyagi AM, Yu M, Darby TM, Vaccaro C, Li JY, Owens JA, et al. The microbial metabolite butyrate stimulates bone formation via t regulatory cell-mediated regulation of wnt10b expression. Immunity. 2018;49:1116–31. doi: 10.1016/j.immuni.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lei H, Schmidt-Bleek K, Dienelt A, Reinke P, Volk HD. Regulatory t cell-mediated anti-inflammatory effects promote successful tissue repair in both indirect and direct manners. Front Pharmacol. 2015;6:184. doi: 10.3389/fphar.2015.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ashcroft AJ, Cruickshank SM, Croucher PI, Perry MJ, Rollinson S, Lippitt JM, et al. Colonic dendritic cells, intestinal inflammation, and t cell-mediated bone destruction are modulated by recombinant osteoprotegerin. Immunity. 2003;19:849–61. doi: 10.1016/s1074-7613(03)00326-1. [DOI] [PubMed] [Google Scholar]
- 134.Levescot A, Chang MH, Schnell J, Nelson-Maney N, Yan J, Martinez-Bonet M, et al. Il-1beta-driven osteoclastogenic tregs accelerate bone erosion in arthritis. J Clin Invest. 2021;131:e141008. doi: 10.1172/JCI141008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Quinn JM, Sims NA, Saleh H, Mirosa D, Thompson K, Bouralexis S, et al. Il-23 inhibits osteoclastogenesis indirectly through lymphocytes and is required for the maintenance of bone mass in mice. J Immunol. 2008;181:5720–9. doi: 10.4049/jimmunol.181.8.5720. [DOI] [PubMed] [Google Scholar]
- 136.Furukawa M, Takaishi H, Takito J, Yoda M, Sakai S, Hikata T, et al. Il-27 abrogates receptor activator of nf-kappa b ligand-mediated osteoclastogenesis of human granulocyte-macrophage colony-forming unit cells through stat1-dependent inhibition of c-fos. J Immunol. 2009;183:2397–406. doi: 10.4049/jimmunol.0802091. [DOI] [PubMed] [Google Scholar]
- 137.Li L, Bhatia R. Stem cell quiescence. Clin Cancer Res. 2011;17:4936–41. doi: 10.1158/1078-0432.ccr-10-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Clezardin P, Coleman R, Puppo M, Ottewell P, Bonnelye E, Paycha F, et al. Bone metastasis: mechanisms, therapies, and biomarkers. Physiol Rev. 2021;101:797–855. doi: 10.1152/physrev.00012.2019. [DOI] [PubMed] [Google Scholar]
- 139.Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L, et al. Bone metastases: an overview. Oncol Rev. 2017;11:321. doi: 10.4081/oncol.2017.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. doi: 10.1016/s0140-6736(00)49915-0. [DOI] [PubMed] [Google Scholar]
- 141.Weilbaecher KN, Guise TA, Mccauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–25. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liang Y, Yi L, Liu P, Jiang L, Wang H, Hu A, et al. Cx3cl1 involves in breast cancer metastasizing to the spine via the src/fak signaling pathway. J Cancer. 2018;9:3603–12. doi: 10.7150/jca.26497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 144.Huergo-Zapico L, Parodi M, Cantoni C, Lavarello C, Fernandez-Martinez JL, Petretto A, et al. Nk-cell editing mediates epithelial-to-mesenchymal transition via phenotypic and proteomic changes in melanoma cell lines. Cancer Res. 2018;78:3913–25. doi: 10.1158/0008-5472.can-17-1891. [DOI] [PubMed] [Google Scholar]
- 145.Ohlsson C, Mellstrom D, Carlzon D, Orwoll E, Ljunggren O, Karlsson MK, et al. Older men with low serum igf-1 have an increased risk of incident fractures: the mros Sweden study. J Bone Miner Res. 2011;26:865–72. doi: 10.1002/jbmr.281. [DOI] [PubMed] [Google Scholar]
- 146.Lincoln J, Alfieri CM, Yutzey KE. Bmp and fgf regulatory pathways control cell lineage diversification of heart valve precursor cells. Dev Biol. 2006;292:292–302. doi: 10.1016/j.ydbio.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 147.Deligiorgi MV, Panayiotidis MI, Siasos G, Trafalis DT. Osteoporosis entwined with cardiovascular disease: the implication of osteoprotegerin and the example of statins. Curr Med Chem. 2021;28:1443–67. doi: 10.2174/0929867327666200123151132. [DOI] [PubMed] [Google Scholar]
- 148.Rochette L, Meloux A, Rigal E, Zeller M, Cottin Y, Vergely C. The role of osteoprotegerin and its ligands in vascular function. Int J Mol Sci. 2019;20:705. doi: 10.3390/ijms20030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tsukasaki M, Asano T, Muro R, Huynh NC, Komatsu N, Okamoto K, et al. Opg production matters where it happened. Cell Rep. 2020;32:108124. doi: 10.1016/j.celrep.2020.108124. [DOI] [PubMed] [Google Scholar]
- 150.Bjerre M. Osteoprotegerin (opg) as a biomarker for diabetic cardiovascular complications. SpringerPlus. 2013;2:658. doi: 10.1186/2193-1801-2-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bjerre M, Munk K, Sloth AD, Nielsen SS, Flyvbjerg A, Botker HE. High osteoprotegerin levels predict macce in stemi patients, but are not associated with myocardial salvage. Scand Cardiovasc J. 2014;48:209–15. doi: 10.3109/14017431.2014.917767. [DOI] [PubMed] [Google Scholar]
- 152.Peng Y, Wu S, Li Y, Crane JL. Type h blood vessels in bone modeling and remodeling. Theranostics. 2020;10:426–36. doi: 10.7150/thno.34126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hankenson KD, Dishowitz M, Gray C, Schenker M. Angiogenesis in bone regeneration. Injury. 2011;42:556–61. doi: 10.1016/j.injury.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–8. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang ZX, Luo ZW, Li FX, Cao J, Rao SS, Liu YW, et al. Aged bone matrix-derived extracellular vesicles as a messenger for calcification paradox. Nat Commun. 2022;13:1453. doi: 10.1038/s41467-022-29191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marshall SM. The pancreas in health and in diabetes. Diabetologia. 2020;63:1962–5. doi: 10.1007/s00125-020-05235-z. [DOI] [PubMed] [Google Scholar]
- 157.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18:427–44. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 158.Funderburgh JL, Corpuz LM, Roth MR, Funderburgh ML, Tasheva ES, Conrad GW. Mimecan, the 25-kda corneal keratan sulfate proteoglycan, is a product of the gene producing osteoglycin. J Biol Chem. 1997;272:28089–95. doi: 10.1074/jbc.272.44.28089. [DOI] [PubMed] [Google Scholar]
- 159.Lee NJ, Ali N, Zhang L, Qi Y, Clarke I, Enriquez RF, et al. Osteoglycin, a novel coordinator of bone and glucose homeostasis. Mol Metabol. 2018;13:30–44. doi: 10.1016/j.molmet.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tasheva ES, Koester A, Paulsen AQ, Garrett AS, Boyle DL, Davidson HJ, et al. Mimecan/osteoglycin-deficient mice have collagen fibril abnormalities. Mol Vis. 2002;8:407–15. [PubMed] [Google Scholar]
- 161.Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab. 2009;94:827–32. doi: 10.1210/jc.2008-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Reinehr T, Roth CL. A new link between skeleton, obesity and insulin resistance: relationships between osteocalcin, leptin and insulin resistance in obese children before and after weight loss. Int J Obes. 2010;34:852–8. doi: 10.1038/ijo.2009.282. [DOI] [PubMed] [Google Scholar]
- 163.Kim YS, Nam JS, Yeo DW, Kim KR, Suh SH, Ahn CW. The effects of aerobic exercise training on serum osteocalcin, adipocytokines and insulin resistance on obese young males. Clin Endocrinol. 2015;82:686–94. doi: 10.1111/cen.12601. [DOI] [PubMed] [Google Scholar]
- 164.Ferron M, Lacombe J. Regulation of energy metabolism by the skeleton: osteocalcin and beyond. Arch Biochem Biophys. 2014;561:137–46. doi: 10.1016/j.abb.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 165.Zee T, Settembre C, Levine RL, Karsenty G. T-cell protein tyrosine phosphatase regulates bone resorption and whole-body insulin sensitivity through its expression in osteoblasts. Mol Cell Biol. 2012;32:1080–8. doi: 10.1128/mcb.06279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yamamoto M, Yamauchi M, Sugimoto T. Elevated sclerostin levels are associated with vertebral fractures in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013;98:4030–7. doi: 10.1210/jc.2013-2143. [DOI] [PubMed] [Google Scholar]
- 167.Guo H, Wang C, Jiang B, Ge S, Cai J, Zhou Y, et al. Association of insulin resistance and beta-cell function with bone turnover biomarkers in dysglycemia patients. Front Endocrinol. 2021;12:554604. doi: 10.3389/fendo.2021.554604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Madsen J, Herskin CW, Zerahn B, Jensen AK, Jorgensen NR, Olsen BS, et al. Bone turnover markers during the remission phase in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2020;21:366–76. doi: 10.1111/pedi.12963. [DOI] [PubMed] [Google Scholar]
- 169.An Y, Liu S, Wang W, Dong H, Zhao W, Ke J, et al. Low serum levels of bone turnover markers are associated with the presence and severity of diabetic retinopathy in patients with type 2 diabetes mellitus. J Diabetes. 2021;13:111–23. doi: 10.1111/1753-0407.13089. [DOI] [PubMed] [Google Scholar]
- 170.Khan MP, Singh AK, Joharapurkar AA, Yadav M, Shree S, Kumar H, et al. Pathophysiological mechanism of bone loss in type 2 diabetes involves inverse regulation of osteoblast function by pgc-1alpha and skeletal muscle atrogenes: adipor1 as a potential target for reversing diabetes-induced osteopenia. Diabetes. 2015;64:2609–23. doi: 10.2337/db14-1611. [DOI] [PubMed] [Google Scholar]
- 171.Hu K, Adachi JD. Glucocorticoid induced osteoporosis. Expet Rev Endocrinol Metabol. 2019;14:259–66. doi: 10.1080/17446651.2019.1617131. [DOI] [PubMed] [Google Scholar]
- 172.Yamamoto M, Yamaguchi T, Yamauchi M, Kaji H, Sugimoto T. Diabetic patients have an increased risk of vertebral fractures independent of bmd or diabetic complications. J Bone Miner Res. 2009;24:702–9. doi: 10.1359/jbmr.081207. [DOI] [PubMed] [Google Scholar]
- 173.Kanazawa I, Tomita T, Miyazaki S, Ozawa E, Yamamoto LA, Sugimoto T. Bazedoxifene ameliorates homocysteine-induced apoptosis and accumulation of advanced glycation end products by reducing oxidative stress in mc3t3-e1 cells. Calcif Tissue Int. 2017;100:286–97. doi: 10.1007/s00223-016-0211-x. [DOI] [PubMed] [Google Scholar]
- 174.Kim OY, Song J. The importance of bdnf and rage in diabetes-induced dementia. Pharmacol Res. 2020;160:105083. doi: 10.1016/j.phrs.2020.105083. [DOI] [PubMed] [Google Scholar]
- 175.Takagi R, Sakamoto E, Kido JI, Inagaki Y, Hiroshima Y, Naruishi K, et al. S100a9 increases il-6 and rankl expressions through mapks and stat3 signaling pathways in osteocyte-like cells. BioMed Res Int. 2020;2020:7149408. doi: 10.1155/2020/7149408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang N, Xu P, Wang X, Yao W, Wang B, Wu Y, et al. Timosaponin aiii attenuates inflammatory injury in ages-induced osteoblast and alloxan-induced diabetic osteoporosis zebrafish by modulating the rage/mapk signaling pathways. Phytomedicine. 2020;75:153247. doi: 10.1016/j.phymed.2020.153247. [DOI] [PubMed] [Google Scholar]
- 177.Ogawa N, Yamaguchi T, Yano S, Yamauchi M, Yamamoto M, Sugimoto T. The combination of high glucose and advanced glycation end-products (ages) inhibits the mineralization of osteoblastic mc3t3-e1 cells through glucose-induced increase in the receptor for ages. Horm Metab Res. 2007;39:871–5. doi: 10.1055/s-2007-991157. [DOI] [PubMed] [Google Scholar]
- 178.Notsu M, Yamaguchi T, Okazaki K, Tanaka K, Ogawa N, Kanazawa I, et al. Advanced glycation end product 3 (age3) suppresses the mineralization of mouse stromal st2 cells and human mesenchymal stem cells by increasing tgf-beta expression and secretion. Endocrinology. 2014;155:2402–10. doi: 10.1210/en.2013-1818. [DOI] [PubMed] [Google Scholar]
- 179.Conte C, Epstein S, Napoli N. Insulin resistance and bone: a biological partnership. Acta Diabetol. 2018;55:305–14. doi: 10.1007/s00592-018-1101-7. [DOI] [PubMed] [Google Scholar]
- 180.Lanham SA, Blache D, Oreffo R, Fowden AL, Forhead AJ. Pancreas deficiency modifies bone development in the ovine fetus near term. J Endocrinol. 2021;252:71–80. doi: 10.1530/JOE-21-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cipriani C, Colangelo L, Santori R, Renella M, Mastrantonio M, Minisola S, et al. The interplay between bone and glucose metabolism. Front Endocrinol. 2020;11:122. doi: 10.3389/fendo.2020.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Thomas DM, Hards DK, Rogers SD, Ng KW, Best JD. Insulin receptor expression in bone. J Bone Miner Res. 1996;11:1312–20. doi: 10.1002/jbmr.5650110916. [DOI] [PubMed] [Google Scholar]
- 183.Sekulovski N, Whorton AE, Shi M, Hayashi K, Maclean JN. Insulin signaling is an essential regulator of endometrial proliferation and implantation in mice. Faseb J. 2021;35:e21440. doi: 10.1096/fj.202002448R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Faienza MF, Luce V, Ventura A, Colaianni G, Colucci S, Cavallo L, et al. Skeleton and glucose metabolism: a bone-pancreas loop. Int J Endocrinol. 2015;2015:758148. doi: 10.1155/2015/758148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fulzele K, Riddle RC, Digirolamo DJ, Cao X, Wan C, Chen D, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2022;185:746. doi: 10.1016/j.cell.2022.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Oh JH, Lee NK. Up-regulation of rank expression via erk1/2 by insulin contributes to the enhancement of osteoclast differentiation. Mol Cells. 2017;40:371–7. doi: 10.14348/molcells.2017.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, et al. Insulin-regulated hepatic gluconeogenesis through foxo1-pgc-1alpha interaction. Nature. 2003;423:550–5. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 188.Figurek A, Rroji M, Spasovski G. Sclerostin: a new biomarker of ckd-mbd. Int Urol Nephrol. 2020;52:107–13. doi: 10.1007/s11255-019-02290-3. [DOI] [PubMed] [Google Scholar]
- 189.Neto R, Pereira L, Magalhaes J, Quelhas-Santos J, Martins S, Carvalho C, et al. Sclerostin and dkk1 circulating levels associate with low bone turnover in patients with chronic kidney disease stages 3 and 4. Clin Kidney J. 2021;14:2401–8. doi: 10.1093/ckj/sfab081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zeng C, Guo C, Cai J, Tang C, Dong Z. Serum sclerostin in vascular calcification and clinical outcome in chronic kidney disease. Diab Vasc Dis Res. 2018;15:99–105. doi: 10.1177/1479164117742316. [DOI] [PubMed] [Google Scholar]
- 191.Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, et al. Fgf-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112:683–92. doi: 10.1172/jci18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Quarles LD. Role of fgf23 in vitamin d and phosphate metabolism: implications in chronic kidney disease. Exp Cell Res. 2012;318:1040–8. doi: 10.1016/j.yexcr.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ryan ZC, Ketha H, Mcnulty MS, Mcgee-Lawrence M, Craig TA, Grande JP, et al. Sclerostin alters serum vitamin d metabolite and fibroblast growth factor 23 concentrations and the urinary excretion of calcium. Proc Natl Acad Sci U S A. 2013;110:6199–204. doi: 10.1073/pnas.1221255110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Brandenburg VM, Kramann R, Koos R, Kruger T, Schurgers L, Muhlenbruch G, et al. Relationship between sclerostin and cardiovascular calcification in hemodialysis patients: a cross-sectional study. BMC Nephrol. 2013;14:219. doi: 10.1186/1471-2369-14-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cejka D, Herberth J, Branscum AJ, Fardo DW, Monier-Faugere MC, Diarra D, et al. Sclerostin and dickkopf-1 in renal osteodystrophy. Clin J Am Soc Nephrol. 2011;6:877–82. doi: 10.2215/cjn.06550810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Thambiah S, Roplekar R, Manghat P, Fogelman I, Fraser WD, Goldsmith D, et al. Circulating sclerostin and dickkopf-1 (dkk1) in predialysis chronic kidney disease (ckd): relationship with bone density and arterial stiffness. Calcif Tissue Int. 2012;90:473–80. doi: 10.1007/s00223-012-9595-4. [DOI] [PubMed] [Google Scholar]
- 197.Morena M, Jaussent I, Dupuy AM, Bargnoux AS, Kuster N, Chenine L, et al. Osteoprotegerin and sclerostin in chronic kidney disease prior to dialysis: potential partners in vascular calcifications. Nephrol Dial Transplant. 2015;30:1345–56. doi: 10.1093/ndt/gfv081. [DOI] [PubMed] [Google Scholar]
- 198.Cozzolino M, Mazzaferro S. The fibroblast growth factor 23: a new player in the field of cardiovascular, bone and renal disease. Curr Vasc Pharmacol. 2010;8:404–11. doi: 10.2174/157016110791112313. [DOI] [PubMed] [Google Scholar]
- 199.Kuro -OM, Moe OW. Fgf23-alphaklotho as a paradigm for a kidney-bone network. Bone. 2017;100:4–18. doi: 10.1016/j.bone.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 200.Cozzolino M, Gentile G, Mazzaferro S, Brancaccio D, Ruggenenti P, Remuzzi G. Blood pressure, proteinuria, and phosphate as risk factors for progressive kidney disease: a hypothesis. Am J Kidney Dis. 2013;62:984–92. doi: 10.1053/j.ajkd.2013.02.379. [DOI] [PubMed] [Google Scholar]
- 201.Titchenell PM, Chu Q, Monks BR, Birnbaum MJ. Hepatic insulin signalling is dispensable for suppression of glucose output by insulin in vivo. Nat Commun. 2015;6:7078. doi: 10.1038/ncomms8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Musso G, Paschetta E, Gambino R, Cassader M, Molinaro F. Interactions among bone, liver, and adipose tissue predisposing to diabesity and fatty liver. Trends Mol Med. 2013;19:522–35. doi: 10.1016/j.molmed.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 203.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339–50. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 204.Scorletti E, Afolabi PR, Miles EA, Smith DE, Almehmadi A, Alshathry A, et al. Design and rationale of the insyte study: a randomised, placebo controlled study to test the efficacy of a synbiotic on liver fat, disease biomarkers and intestinal microbiota in non-alcoholic fatty liver disease. Contemp Clin Trials. 2018;71:113–23. doi: 10.1016/j.cct.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 205.Yilmaz Y. Review article: non-alcoholic fatty liver disease and osteoporosis--clinical and molecular crosstalk. Aliment Pharmacol Ther. 2012;36:345–52. doi: 10.1111/j.1365-2036.2012.05196.x. [DOI] [PubMed] [Google Scholar]
- 206.Nussler AK, Wildemann B, Freude T, Litzka C, Soldo P, Friess H, et al. Chronic ccl4 intoxication causes liver and bone damage similar to the human pathology of hepatic osteodystrophy: a mouse model to analyse the liver-bone axis. Arch Toxicol. 2014;88:997–1006. doi: 10.1007/s00204-013-1191-5. [DOI] [PubMed] [Google Scholar]
- 207.Lu K, Shi TS, Shen SY, Shi Y, Gao HL, Wu J, et al. Defects in a liver-bone axis contribute to hepatic osteodystrophy disease progression. Cell Metabol. 2022;34:441–57. doi: 10.1016/j.cmet.2022.02.006. [DOI] [PubMed] [Google Scholar]
- 208.Zhang ZH, Jia XY, Fang JY, Chai H, Huang Q, She C, et al. Reduction of sost gene promotes bone formation through the wnt/beta-catenin signalling pathway and compensates particle-induced osteolysis. J Cell Mol Med. 2020;24:4233–44. doi: 10.1111/jcmm.15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Czepiel M, Stec M, Korkosz M, Gula Z, Blyszczuk P, Baran J, et al. Down-regulation of dkk-1 in platelets of patients with axial spondyloarthritis. Arthritis Rheumatol. 2021;73:1831–4. doi: 10.1002/art.41739. [DOI] [PubMed] [Google Scholar]
- 210.Polyzos SA, Anastasilakis AD, Kountouras J, Makras P, Papatheodorou A, Kokkoris P, et al. Circulating sclerostin and dickkopf-1 levels in patients with nonalcoholic fatty liver disease. J Bone Miner Metab. 2016;34:447–56. doi: 10.1007/s00774-015-0687-x. [DOI] [PubMed] [Google Scholar]
- 211.Du J, Pan X, Lu Z, Gao M, Hu X, Zhang X, et al. Serum osteocalcin levels are inversely associated with the presence of nonalcoholic fatty liver disease in patients with coronary artery disease. Int J Clin Exp Med. 2015;8:21435–41. [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang XL, Wang YN, Ma LY, Liu ZS, Ye F, Yang JH. Uncarboxylated osteocalcin ameliorates hepatic glucose and lipid metabolism in kkay mice via activating insulin signaling pathway. Acta Pharmacol Sin. 2020;41:383–93. doi: 10.1038/s41401-019-0311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Du J, Zhang M, Lu J, Zhang X, Xiong Q, Xu Y, et al. Osteocalcin improves nonalcoholic fatty liver disease in mice through activation of nrf2 and inhibition of jnk. Endocrine. 2016;53:701–9. doi: 10.1007/s12020-016-0926-5. [DOI] [PubMed] [Google Scholar]
- 214.Basu R, Kulkarni P, Qian Y, Walsh C, Arora P, Davis E, et al. Growth hormone upregulates melanocyte-inducing transcription factor expression and activity via jak2-stat5 and src signaling in gh receptor-positive human melanoma. Cancers. 2019;11:1352. doi: 10.3390/cancers11091352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Pelosi P, Lapi E, Cavalli L, Verrotti A, Pantaleo M, de Martino M, et al. Bone status in a patient with insulin-like growth factor-1 receptor deletion syndrome: bone quality and structure evaluation using dual-energy x-ray absorptiometry, peripheral quantitative computed tomography, and quantitative ultrasonography. Front Endocrinol. 2017;8:227. doi: 10.3389/fendo.2017.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Poinsot P, Schwarzer M, Peretti N, Leulier F. The emerging connections between igf1, the intestinal microbiome, lactobacillus strains and bone growth. J Mol Endocrinol. 2018;61:T103–13. doi: 10.1530/jme-17-0292. [DOI] [PubMed] [Google Scholar]
- 217.Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, et al. Circulating levels of igf-1 directly regulate bone growth and density. J Clin Invest. 2002;110:771–81. doi: 10.1172/jci0215463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.De Toni L, Jawich K, De Rocco PM, Di Nisio A, Foresta C. Osteocalcin: a protein hormone connecting metabolism, bone and testis function. Protein Pept Lett. 2020;27:1268–75. doi: 10.2174/0929866527666200505220459. [DOI] [PubMed] [Google Scholar]
- 219.Manolagas SC, Kousteni S, Jilka RL. Sex steroids and bone. Recent Prog Horm Res. 2002;57:385–409. doi: 10.1210/rp.57.1.385. [DOI] [PubMed] [Google Scholar]
- 220.Lin C, Yu S, Jin R, Xiao Y, Pan M, Pei F, et al. Circulating mir-338 cluster activities on osteoblast differentiation: potential diagnostic and therapeutic targets for postmenopausal osteoporosis. Theranostics. 2019;9:3780–97. doi: 10.7150/thno.34493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Majeska RJ, Ryaby JT, Einhorn TA. Direct modulation of osteoblastic activity with estrogen. J Bone Joint Surg Am. 1994;76:713–21. doi: 10.2106/00004623-199405000-00013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material