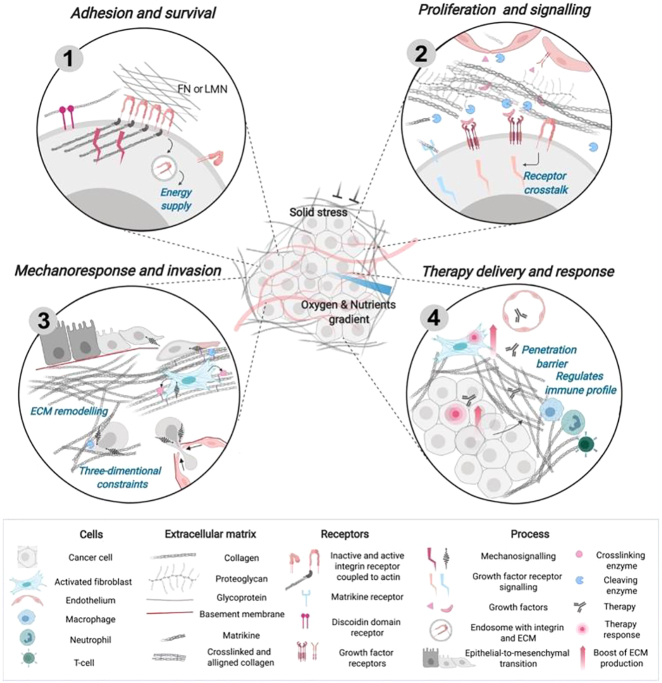
Figure 1:
The ECM as a regulator of tumor progression and therapy.
(1) Adhesion to the ECM through the receptors (e.g., via integrin or discoidin domain receptor) promotes signaling that supports cancer cell survival. To supply metabolic pathways, cancer cells can use the ECM (laminin and fibronectin), internalized with an integrin. (2) Growth factors that are released upon cleavage by ECM proteases (mainly MMPs) can regulate cancer cells and other cells of the TME. Biologically active ECM fragments (peptides) released upon proteolysis can promote cell signaling in the TME and spread with the blood flow. (3) Recruitment of activated fibroblasts stimulates ECM production, alignment, and cross‐linking (e.g., by LOX) of collagen fibrils resulting in fibrosis. Changes in mechanical properties can prompt cancer cells to adapt their signaling, gene expression, and cytoskeleton polarization, acquiring invasive phenotype (EMT). (4) Dense and rigid ECM creates a barrier to drug and T-cell penetration. Targeted cancer cells and fibroblasts can increase their resistance by promoting ECM production and deposition. Changes in the tumor ECM are associated with the formation of a tumor‐permissive immune profile (Figure reproduced with permission [42]).