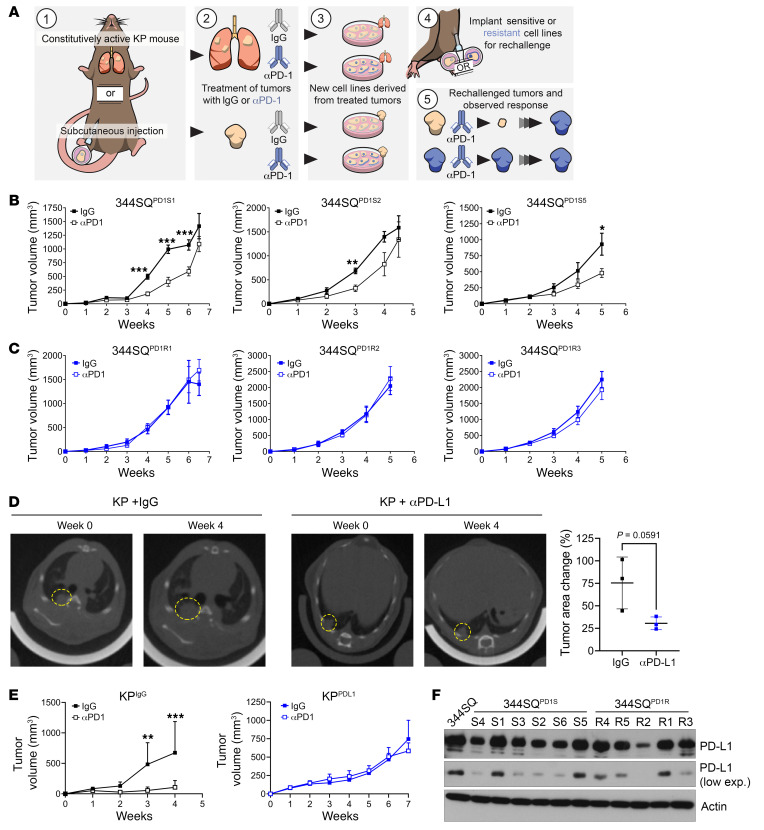
Figure 1. Tumor models created from KP subcutaneous tumors or GEMM lung tumors treated with anti–PD-(L)1 display intrinsic resistance when rechallenged in vivo.
(A) Schematic illustrating the development of anti–PD-1– or anti–PD-L1–resistant KP tumor models. Tumors were generated either with subcutaneous implantation models using syngeneic 344SQ KP murine lung cancer cells or from autochthonous lung tumors developed in the KrasLA1-G12D/p53R172HΔg GEMM. Mice were then treated with IgG control or PD-1/PD-L1 axis–blocking antibodies until the development of resistance. At this point, tumors were excised, cultured, and expanded ex vivo, and then reimplanted into wild-type (WT) mice for rechallenge with anti–PD-(L1). (B) Three of the 344SQ IgG-treated tumors described in A (344SQPD1S) were implanted into WT mice and treated with either IgG or anti–PD-1. Tumors were measured weekly with calipers. n = 5 mice per group. *P < 0.05, **P < 0.01, ***P < 0.001 by multiple t tests (1 per time point). (C) The anti–PD-1–treated tumors described in A (344SQPD1R) were implanted and treated as in B. (D) KrasLA1-G12D/p53R172HΔg mice were imaged by micro-CT to confirm lung nodule formation. Mice were randomly distributed into IgG or anti–PD-L1 treatment arms and treated for 4 weeks. Endpoint images using micro-CT were taken (left). The percentage change in tumor area was measured for 3 independent tumors per mouse (right). (E) Cell lines were derived from the IgG-treated (KPIgG) or anti–PD-L1–treated (KPPDL1) GEMMs described in D and implanted into WT mice. Mice were rechallenged with anti–PD-L1 or IgG control antibodies and tumor response measured over time using calipers. n = 5 mice per group. **P < 0.01, ***P < 0.001 by multiple t tests (1 per time point). (F) 344SQPD1S and 344SQPD1R cells were analyzed for PD-L1 expression by Western blotting (see supplemental material for full, uncut gels). Actin was used as a loading control.