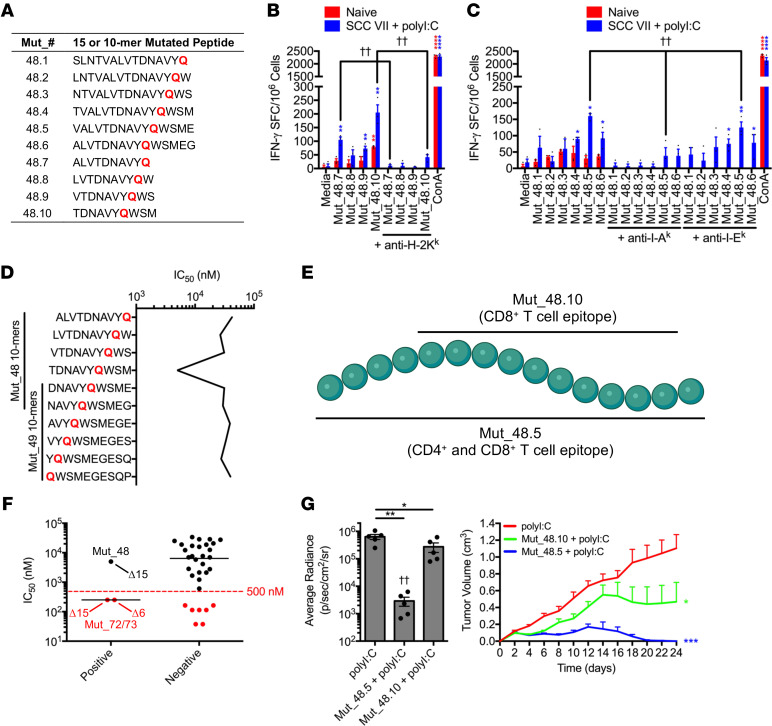
Figure 4. MHC restriction and functional interplay of CD4+ and CD8+ T cell vaccine-derived epitopes.
(A) H129Q 15/10-mer peptides derived from Mut_48. (B and C) Naive C3H/HeJ mice compared with animals that received a 1 × 107 irradiated SCC VII cell and 50 μg polyI:C immunization followed by a 5 × 105 live SCC VII-Luc/GFP cell challenge at day 14. Day 28 splenic/Ig LN (B) CD8+ T cells and (C) CD4+ T cells cocultured with Mut_48-derived minimal peptide-pulsed BMDCs for quantification of IFN-γ-producing cells via ELISPOT ± blocking antibodies against I-Ak, I-Ek, and H-2Kk (n = 3 per group). (D) IEDB NetMHCpan (v4.0) MHC I predictions of minimal peptide binding to murine H-2Kk. (E) Mut_48.10 and Mut_48.5 epitope schematic. (F) CD8+ T cell ELISPOT responses against Pool_9, Pool_10, Pool_11, Pool_13, Pool_14, and Pool_15 clustered by IFN-γ production (positive versus negative). Represented are IEDB NetMHCpan (v4.0) MHC I predictions of minimal peptide binding to murine H-2Kk. (G) C3H/HeJ mice vaccinated with 50 μg polyI:C alone or in combination with 5 μg Mut_48.5 or Mut_48.10 peptides in a booster regimen 21 days apart followed by challenge with 5 × 105 live SCC VII-Luc/GFP cells 31 days after primary vaccination. Day 14 bioluminescence and tumor volume kinetics (n = 5–6 per group). All experiments were performed 2 or more times and data indicate mean ± (B, C, and G) SEM or (F) median; (G, bioluminescence) *P < 0.05 and **P < 0.01 (Student’s t test); ††P < 0.01 (1-way ANOVA and Dunnett’s posthoc test relative to polyI:C); (G, tumor volume) *P < 0.05 and ***P < 0.001 (2-way ANOVA and Dunnett’s posthoc test relative to polyI:C); (B and C) *P < 0.05, **P < 0.01, and ****P < 0.0001 (Student’s t test of data with SI > 2 and Poisson < 5%); (B) ††P < 0.01 (Student’s t test); (C) ††P < 0.01 (1-way ANOVA and multiple comparison Tukey’s posthoc test).