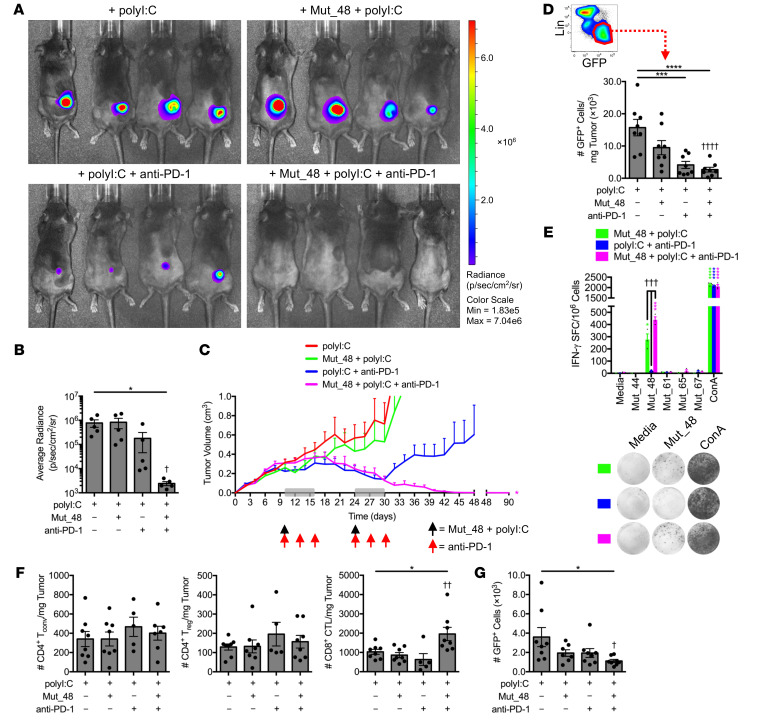
Figure 7. Delayed therapeutic codelivery of anti-PD-1 and Cltc.
Δ15 promotes clearance of established SCC VII tumors. C3H/HeJ mice injected with 5 × 105 live SCC VII-Luc/GFP cells and given 50 μg polyI:C alone or in combination with 5 μg Mut_48 peptide at day 10 after challenge (black arrow). Select groups of mice also received anti-PD-1 at days 10, 13, and 16 (red arrow). The immunotherapy cycle repeated at day 24 (gray box). (A and B) Bioluminescence of mice at 35 days after challenge and (C) tumor volume kinetics tracked to day 90 (n = 5–6 per group). (D) Tumors harvested at day 17 assessed for Lin−GFP+ SCC VII cells where Lin (lineage) comprised a dump gate of anti-CD31, anti-CD45, and anti-LYVE1 (n = 8 per group). (E) Mononuclear cells harvested from the spleens and Ig LNs of surviving C3H/HeJ mice at day 90 after live-cell challenge assessed for IFN-γ production via ELISPOT after restimulation with NeoAg-pulsed BMDCs (n = 3 per group). (F) Day 17 TIL assessed for CD4+CD25±FoxP3− Tconv, CD4+CD25+FoxP3+ Treg, and CD8+ CTL (n = 5–8 per group). (G) Number of total Lin−GFP+ SCC VII cells in the ipsilateral Ig LN from day 17 tumor-bearing mice given therapy beginning at day 10 as a single cycle (n = 7–8 per group). All experiments were performed 2 or more times and data indicate mean ± SEM; (B, D, F, and G) *P < 0.05, ***P < 0.001, and ****P < 0.0001 (Student’s t test); †P < 0.05, ††P < 0.01, and ††††P < 0.0001 (1-way ANOVA and Dunnett’s posthoc test relative to polyI:C); (C) *P < 0.05 (2-way ANOVA and Dunnett’s posthoc test relative to polyI:C); (E) *P < 0.05, ***P < 0.001, and ****P < 0.0001 (Student’s t test of data with SI > 2 and Poisson < 5%); †††P < 0.01 (1-way ANOVA and Dunnett’s posthoc test relative to polyI:C + anti-PD-1).