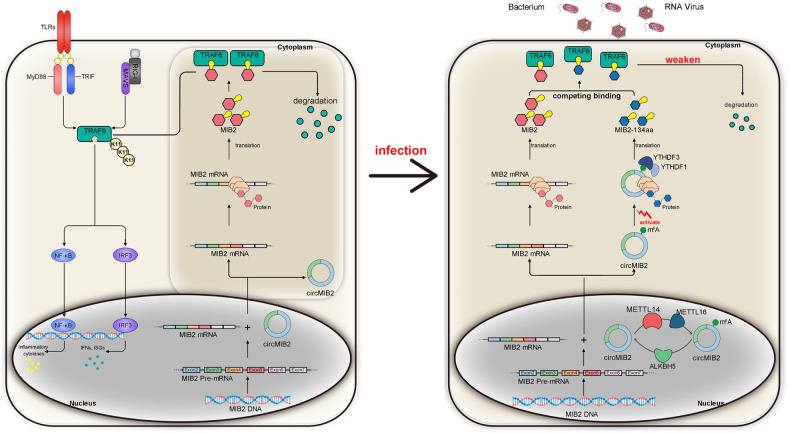
Fig. 8. Schematic diagram of the mechanism MIB2-134aa competes with MIB2 for binding TRAF6 to regulate the ubiquitination.
Under normal circumstances, circMIB2 does not translate to produce proteins. When SCRV virus or V. anguillarum infects the host, the pathway of circMIB2 translating proteins is activated. MIB2 could promote the TRAF6 protein degradation and repress TRAF6-mediated antiviral responses, thereby regulating viral replication and bacterial invasion. MIB2 promoted K11-linked ubiquitination of TRAF6, thereby inhibiting the antiviral responses and helping the virus escape. In addition, MIB2-134aa has the same domain as MIB2 protein. Through this domain, MIB2-134aa can compete with MIB2 for binding to the same position of TRAF6, thereby inhibiting the ubiquitination of TRAF6 and promoting the host’s innate immune response.