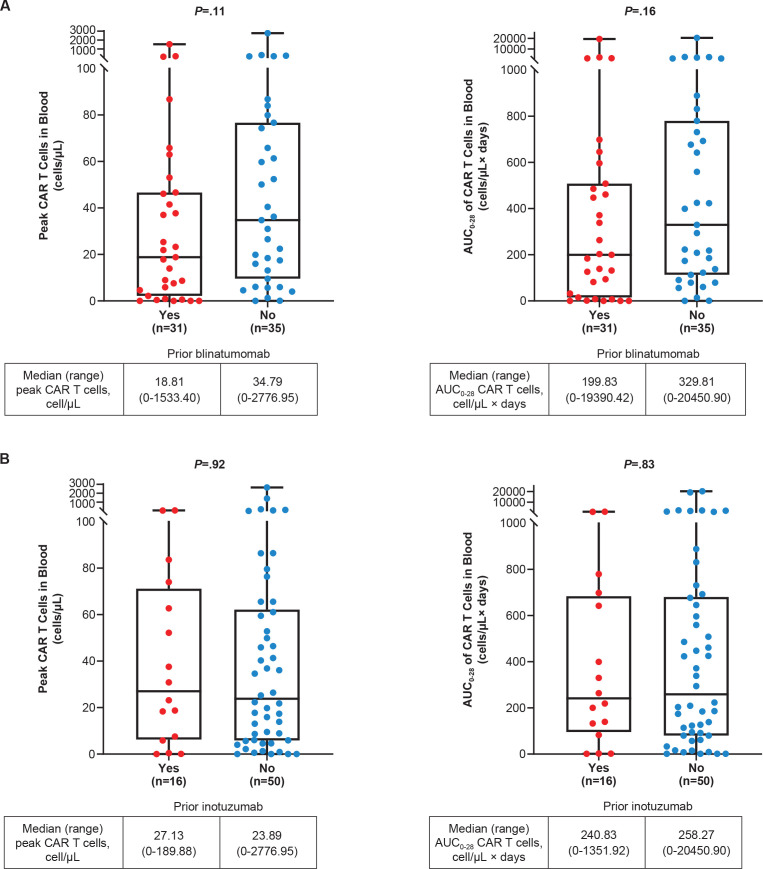
Figure 4.
Peak and AUC0−28 CAR T-cell levels in pooled Phase 1 and 2 treated patients by (A) prior blinatumomab a and (B) prior inotuzumab. b Peak and AUC0−28 CAR T-cell levels assessed in the blood post brexu-cel infusion in pooled Phase 1 and 2 patients by prior blinatumomab (A) and prior inotuzumab (B). Subgroup n is the number of patients with documented CAR T cells in blood. Peak is defined as the maximum number of CAR T cells in blood measured after infusion. AUC0−28 is defined as the AUC in a plot of number of CAR T cells in blood against scheduled visit from Day 0 to Day 28. P value is calculated by Wilcoxon rank-sum test, a rank-based non-parametric test for two groups. a A similar proportion of patients with and without prior blinatumomab had >75% BM blasts at baseline (39% vs 34%, respectively). b For patients with and without prior inotuzumab, the proportion of patients with >75% BM blasts at baseline was 50% and 29%, respectively. AUC0−28, area under the curve from time of dose to 28 days; BM, bone marrow; CAR, chimeric antigen receptor.