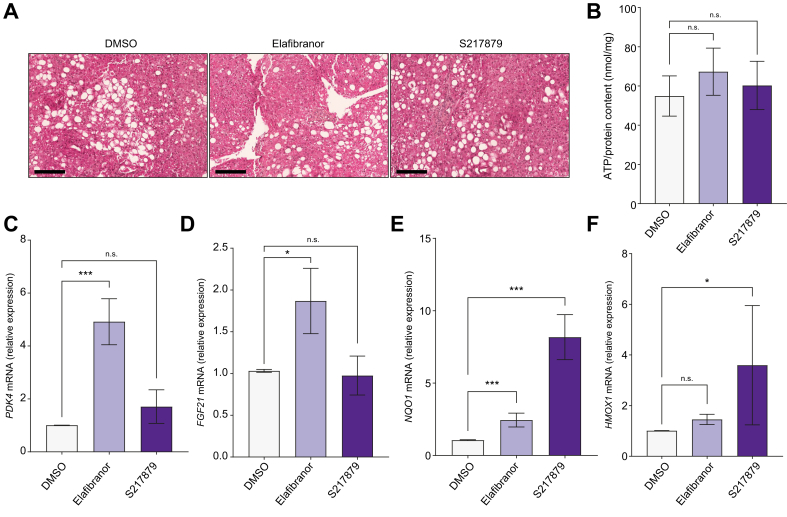
Fig. 1.
Drug safety and efficacy.
Human PCLS were generated from the liver of patients with MAFLD (n = 12) and treated with elafibranor (10 μM) or S217879 (3 μM) or vehicle (DMSO, 0.1%) for 2 days. (A) Representative images of H&E staining of human PCLS used for histological analysis of slice morphology. Scale bar: 200 μm. (B) Quantification of ATP content in human PCLS (ATP/protein content ratio). (C) qPCR analysis of PPARα/δ target genes expression PDK4 and (D) FGF21. (E) qPCR analysis of NRF2 target genes expression NQO1 and (F) HMOX1. Data are expressed as mean ± SEM. ∗p <0.05; ∗∗∗p <0.001; ns, not significant (Wilcoxon paired t test). FGF21, fibroblast growth factor 21; HMOX1, heme oxygenase 1, MAFLD, metabolic-associated fatty liver disease; NQO1, NAD(P)H quinone dehydrogenase 1; NRF2, nuclear factor (erythroid-derived 2)-like 2; PCLS, precision cut liver slices; PDK4, pyruvate dehydrogenase kinase 4; PPAR, peroxisome proliferator-activated receptor; qPCR, quantitative PCR.