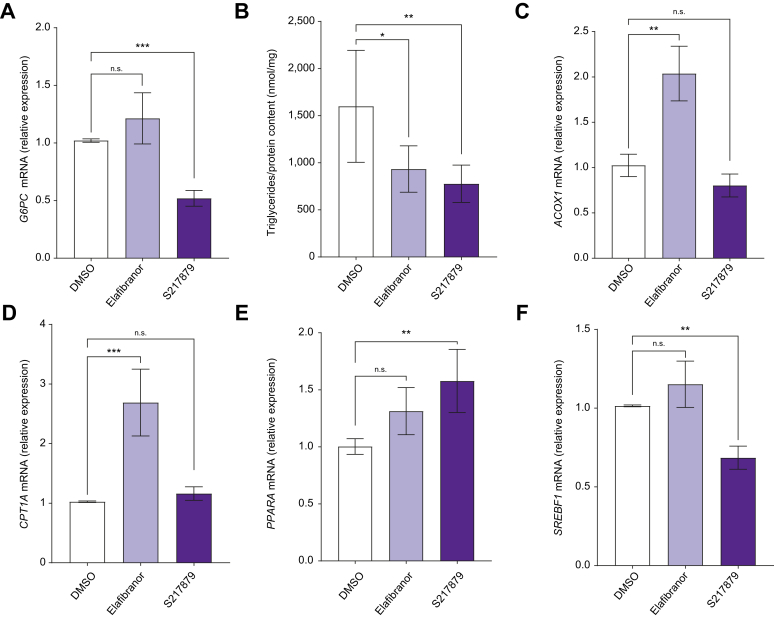
Fig. 2.
Effects of elafibranor and S217879 treatments on liver metabolic features.
Human PCLS were generated from the liver of patients with MAFLD (n = 12) and treated with elafibranor (10 μM) or S217879 (3 μM) or vehicle (DMSO, 0.1%) for 2 days. (A) qPCR analysis of G6PC expression in human PCLS. (B) Quantification of triglycerides content in human PCLS (triglycerides/protein content ratio). (C) qPCR analysis of ACOX1, (D) CPT1A, (E) PPARA and (F) SREBF1 expression in human PCLS. Data are expressed as mean ± SEM. ∗p <0.05; ∗∗p <0.01; ∗∗∗p <0.001; ns, not significant (Wilcoxon paired t test). ACOX1, peroxisomal acyl-coenzyme A oxidase 1; CPT1A, carnitine palmitoyl transferase 1 alpha; G6PC, glucose-6-phosphatase; MAFLD, metabolic-associated fatty liver disease; PCLS, precision cut liver slices; PPARA, peroxisome proliferator-activated receptor-alpha; qPCR, quantitative PCR; SREBF1, sterol regulatory element-binding protein-1.