Figure 3.
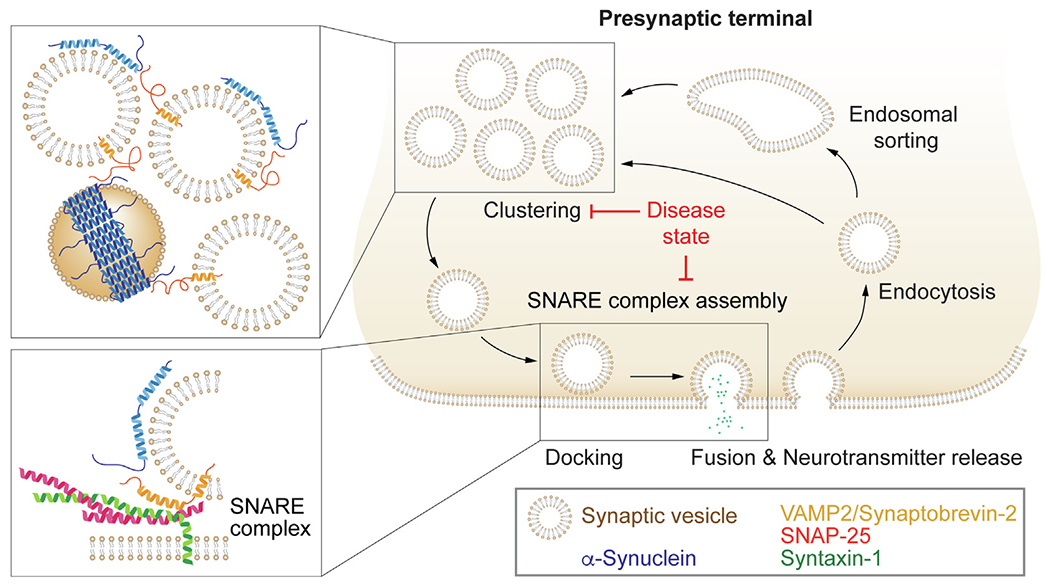
SNARE-dependent effects of α-synuclein on the synaptic vesicle cycle. Upon initiation of an action potential in the presynaptic terminal, synaptic vesicles docked to the plasma membrane fuse to release neurotransmitters to propagate the signal to the next neuron. Synaptic vesicle fusion is mediated by formation of the synaptic SNARE complex, composed of syntaxin-1 and SNAP-25 on the presynaptic plasma membrane, and VAMP2/synaptobrevin-2 on the synaptic vesicle. Following fusion, synaptic vesicle constituents are retrieved via endocytosis. Simultaneous binding of α-synuclein multimers to synaptic vesicle membranes and the synaptic vesicle protein VAMP2/synaptobrevin-2 clusters synaptic vesicles (top inset) and chaperones SNARE complex assembly (bottom inset). In diseased states, pathological α-synuclein or lack of physiologically functional α-synuclein due to recruitment into pathological α-synuclein aggregates results in impaired or dysfunctional synaptic vesicle clustering and reduced SNARE complex assembly. This imbalance in the synaptic vesicle cycle affects long-term neuron function and survival.
