ABSTRACT
Background
Complications of urogenital schistosomiasis include acute inflammatory and chronic fibrotic changes within the urogenital tract. Disease burden of this neglected tropical disease is often underestimated, as only active, urine egg-patent Schistosoma infection is formally considered. Previous studies have focussed on short-term effects of praziquantel treatment on urinary tract pathology, demonstrating that acute inflammation is reversible. However, the reversibility of chronic changes is less well studied.
Methods
Our study compared, at two time points 14 y apart, urine egg-patent infection and urinary tract pathology in a cohort of women living in a highly endemic area having intermittent praziquantel treatment(s). In 2014 we matched 93 women to their findings in a previous study in 2000.
Results
Between 2000 and 2014 the rate of egg-patent infection decreased from 34% (95% confidence interval [CI] 25 to 44) to 9% (95% CI 3 to 14). However, urinary tract pathology increased from 15% (95% CI 8 to 22) to 19% (95% CI 11 to 27), with the greatest increase seen in bladder thickening and shape abnormality.
Conclusions
Despite praziquantel treatment, fibrosis from chronic schistosomiasis outlasts the presence of active infection, continuing to cause lasting morbidity. We suggest that future efforts to eliminate persistent morbidity attributable to schistosomiasis should include intensified disease management.
Keywords: Schistosoma haematobium; schistosomiasis, ultrasonography; urogenital diseases
Introduction
Schistosomiasis continues to be the one of the world's most prevalent parasitic diseases, with an estimated 700 million people at risk and 251 million requiring preventative treatment in 2021.1 Currently sanctioned schistosomiasis surveillance (based on microscopic egg detection) accounts for those with active infection but does not include all those with the persistent sequelae that follow from repeated or prolonged infection. Recently there has been a redirection of schistosomiasis morbidity control away from a focus on infection rates to instead consider the long-lasting morbidity associated with the disease, including the post-infection period. Although the global burden of schistosomiasis-related disease is currently estimated to be 1.4 million disability-adjusted life-years (DALYs) based on active infection prevalence,2 its burden could be as high as 56 million DALYs when long-standing morbidity is taken into consideration.3
Human infection by the waterborne trematode parasite Schistosoma haematobium causes localised damage to the urinary system and genital tract,4 as well as systemic manifestations such as anaemia, growth stunting and issues affecting women's and men's sexual and reproductive health.5 Hosts become infected through contact with schistosome cercariae found in snail-infested fresh water bodies. Adult trematode pairs can release thousands of eggs that are excreted through the host's urinary tract. However, only about half the parasite eggs leave the human body. Pathological changes occur due to the host's immune response to trapped schistosome eggs that remain lodged in the walls of the urinary tract. A polarised T helper cell type 2 (Th2) response initiated by schistosome soluble egg antigen causes an influx of inflammatory cells such as eosinophils, monocytes, fibroblasts and mast cells into the local area.6 Granulation tissue and scarring then forms around trapped eggs in order to prevent further localised egg-induced damage.7 At this stage, S. haematobium–associated inflammation can cause bladder changes, including masses and ulceration, which may manifest as pain, dysuria and haematuria.4,8 Immunoregulation during chronic infection can eventually lead to a dampening (modulation) of the host's immune response, but meanwhile can leave irreversible fibrosis and calcification of the bladder wall or distal ureters. In severe cases, this thickening can lead to ureteric dilatation and renal obstruction as late signs of pathology.9,10 Life-threatening pathology such as renal failure or squamous cell carcinoma of the bladder develops in around 1% of individuals in highly endemic settings.11
The introduction of portable ultrasound imaging has increased our understanding of the evolution of organ-specific pathology in schistosomiasis.12,13 In an attempt to standardise the reporting of pathological changes, the World Health Organization (WHO) has produced a practical guide to ultrasound assessment of both intestinal and urogenital schistosomiasis.14,15 Ultrasound-based community surveys can now be used to assess the prevalence of urinary tract pathology and to monitor the response to praziquantel treatment. While praziquantel is effective for treating active schistosomiasis infection, the treatment-related reversibility of associated urinary tract structural morbidity is still unclear. Owing to practical and temporal constraints, most cohort studies have focused on a short 12- to 24-month follow-up time after praziquantel treatment.16–18 The general consensus is that early inflammatory bladder changes such as masses are reversible with timely treatment, particularly if there is no risk of reinfection,19 whereas chronic changes such as calcification of the bladder, hydroureter and/or hydronephrosis are much less likely to resolve.17,18,20–25 A recent systematic review showed that the greatest impact of praziquantel in reducing bladder pathology was seen when follow-up was within 6 months, whereas the chances of finding reversal of pathology decreased as the time to follow-up increased.26 It has also been suggested that urinary tract pathology in adults is less responsive to praziquantel treatment than the pathology seen in children.18
The Msambweni region of southeastern coastal Kenya is endemic for S. haematobium. Community-based research and control initiatives have been conducted in this area for >30 y. Annual school-based treatment with praziquantel was given between 1984 and 1992 and community-wide treatment has since been given in 2000, 2003 and 2009 as part of ongoing research in the area. Annual treatment of school-aged children continues at present. At the initiation of the control programme, the prevalence of S. haematobium infection was 66% in school-age children,27 and studies in this district have shown a significant reduction in the prevalence and intensity of infection after initiation of annual praziquantel treatment.27,28
The aim of the present study was to use portable ultrasound to describe the current prevalence of schistosomiasis-related urinary tract pathology among adults in a treated community that had previously experienced high rates of active urogenital S. haematobium infection. We also aimed to compare urinary tract pathology in a cohort of women examined at two time points 14 y apart following implementation of community-based control.
Methods
Data for the present study were collected between May and July 2014 in the rural villages of Nganja and Milalani, located at 4.5°S and 39.5°E, within the Msambweni region of Kwale County on the coast of Kenya (Figure 1). At the last census, in 2013, the two village populations were 836 and 1940, respectively. This study was part of ongoing community-based schistosomiasis research within the district.
Figure 1.
Map showing the location of the Msambweni region of coastal Kenya. Source: Bustinduy AL, Thomas CL, Fiutem JJ et al. Measuring fitness of Kenyan children with polyparasitic infections using the 20-meter shuttle run test as a morbidity metric. PLoS Negl Trop Dis 2011;5(7):e1213. Used with permission under Creative Commons Attribution License.
Each village was split into 10 areas to ensure participation from households in all sectors of the study villages. Each day, field workers canvassed 1 of the 10 areas and invited a convenience sample of approximately 30 eligible women to participate in the study. Exclusion criteria were acute febrile illness, inability to give informed consent and suspected or confirmed pregnancy.29 Males and children (<18 y of age) were excluded, as the majority of these groups were away from the village during working hours and therefore a representative sample could not be guaranteed for those subgroups. Demographic information, including participant name, date of birth and household number, was gathered by questionnaire to allow for identification of subjects who had participated in previous 2000-era ultrasound surveys.30
Parasitological testing
Urine samples were collected between the hours of 10:00 h and 14:00 h to coincide with peak egg excretion.31 Egg count was assessed using a standard filtration method as previously described:32 10 ml of urine was passed through a 10-μm polycarbonate filter (Millipore, Livingston, UK) that was then fixed onto a microscope slide and one drop of iodine was placed on the filter. The slide was examined under light microscopy at 40× magnification. The intensity of infection was classified according to standard convention,32 i.e. 1–50 eggs/10 ml was considered a light infection and >50 eggs/10 ml was considered a heavy infection. Parasitological testing was performed after the ultrasound to eliminate observer bias during interpretation of the images.
Ultrasound assessment
Ultrasound assessments of the urinary tract were performed by a highly experienced study ultrasonographer from the Kenya Medical Research Institute CCR Radiology Unit (EI) and the study doctor (KM) using a portable CTS 7700 PLUS machine and 3.5–5 MHz convex array probe (SIUI, Shantou, China). Quality assurance and final interpretation was provided by the study leader (EJ). Findings were recorded and scored using WHO standardised criteria for ultrasound of urogenital schistosomiasis.15 The presence of bladder wall masses, pseudopolyps, irregularities, bladder wall thickening, abnormal bladder shape, ureteric dilatation and/or hydronephrosis was recorded. Before ultrasound examination, participants were given sufficient fluid to ensure that the bladder was adequately filled. If any ureteric or renal pathology was found, this was reassessed 30 mins after urination to eliminate any false positive results caused by overfilling of the bladder.15
Longitudinal follow-up
Individual-level data from a previous ultrasound survey of the same villages, performed in 2000, were made available by FMM, PM and CHK.30 A participant was considered properly matched if she had the same identifying criteria for two of three identification categories (name, year of birth, household). In the new (2014) study, a total of 275 women were surveyed. Of these, 102 were tracked and identified as having participated in the earlier study undertaken in 2000. Of those, 93 of the 102 had an ultrasound examination in the previous study. Nine were excluded, either due to pregnancy or because they were under the age of inclusion for treatment in 2000 (see Figure 2).
Figure 2.
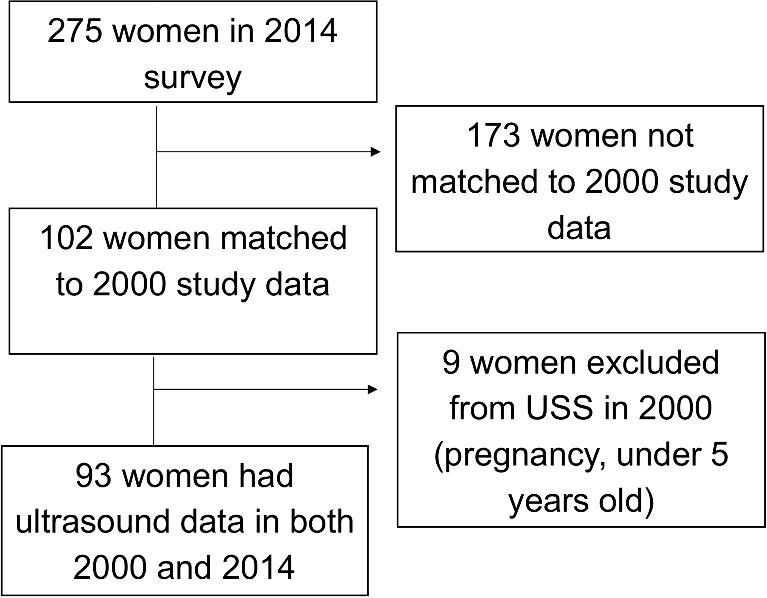
Flow diagram of participants matched to both 2000 and 2014 data for cohort analysis.
Statistical analysis
Information was collected using data collection sheets and was then entered and collated using Excel (Microsoft, Redmond, WA, USA). The data were then analysed using SPSS version 21 (IBM, Armonk, NY, USA). Descriptive analysis and 95% confidence intervals (CIs) were used for comparison of the prevalence of urinary tract abnormalities and of active infection in the 2000 and 2014 data.
RESULTS
Study population demographics
A total of 275 women were included in the 2014 survey: 108 participants from the village of Nganja and 167 from Milalani. The median age of the women was 40.5 years (range 18–84). Table 1 shows the breakdown of egg-patent infection and rates of urinary tract pathology detected in each village.
Table 1.
Demographic data, prevalence of egg-patent infection and urinary pathology among surveyed women in 2014 (N=275).
| Characteristics | Nganja | Milalani |
|---|---|---|
| Participants | 108 | 167 |
| Age (years), median (IQR) | 41.0 (33.5–52.5) | 39.5 (29.5–53.5) |
| Egg-patent infection, n (%) | 9 (8.3) | 10 (6.0) |
| Urinary tract pathology, n (%) | 19 (17.6) | 26 (15.6) |
Prevalence of egg-patent infection and urinary tract pathology in 2014
In 2014, the overall prevalence of active egg-patent schistosomiasis was 6.9%. The infection rates in the two villages were similar (8.3% [9/108]) in Nganja vs 6.0% [10/167] in Milalani). Of those infected, 89% (17/19) had low-intensity egg counts on light microscopy, defined as <50 eggs/10 ml of urine. Of those infected, the geometric mean egg count was 6.3 eggs/10 ml of urine (range 1–368). The arithmetic mean egg count was 44 eggs/10 ml of urine.
Schistosomiasis-related urinary tract abnormalities detected by ultrasound were found in 45/275 (16.4%) women surveyed in 2014. The prevalence of ultrasound abnormalities in the two villages was similar, with 19/108 (17.6%) having detectable abnormalities in Nganja and 26/167 (15.6%) in Milalani. Bladder pathology was much more common than pathology of the upper urinary tract. The breakdown of these and other ultrasound abnormalities detected is shown in Table 2. Examples of urinary tract pathology found on ultrasound are shown in Figure 3. Egg-patent infection was found in 9% (4/45) of women with ultrasound abnormalities compared with 7% (15/229) among women with normal ultrasounds.
Table 2.
Number and prevalence of community-level egg-patent infection and ultrasound-detected urinary tract pathologies among women tested in 2014.
| Characteristics | n | % of total (N=275) | 95% CI, % |
|---|---|---|---|
| S. haematobium prevalence by parasitology | 19 | 6.9 | 3.9 to 9.9 |
| Intensity (per 10 ml of urine) | |||
| Light (1–50) | 17 | 6.2 | 3.9 to 9.7 |
| Heavy (≥50) | 2 | 0.7 | 0.0 to 1.7 |
| Pathology on ultrasound | 45 | 16.4 | 12.0 to 20.8 |
| Bladder pathology | 39 | 14.2 | 10.0 to 18.3 |
| Abnormal shape | 7 | 2.5 | 0.7 to 4.4 |
| Irregularity | 2 | 0.7 | 0.0 to 1.7 |
| Thickening | 33 | 12.0 | 8.1 to 15.9 |
| Mass | 2 | 0.7 | 0.0 to 1.7 |
| Pseudopolyp | 0 | 0.0 | – |
| Upper tract pathology | 8 | 2.9 | 0.9 to 4.9 |
| Unilateral ureteric dilatation | 2 | 0.7 | 0.0 to 1.7 |
| Bilateral ureteric dilatation | 5 | 1.8 | 0.2 to 3.4 |
| Unilateral hydronephrosis | 2 | 0.7 | 0.0 to 1.7 |
| Bilateral hydronephrosis | 2 | 0.7 | 0.0 to 1.7 |
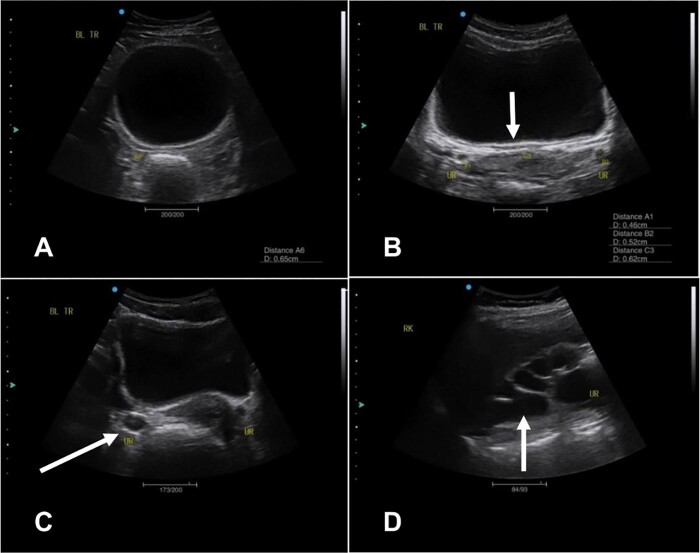
Figure 3.
Ultrasound images showing urinary tract pathology detected in 2014. (A) Transverse image showing an abnormally shaped/rounded bladder. (B) Transverse image of bladder wall thickening. (C) Transverse image showing ureteric dilatation. (D) A sagittal image of the right kidney showing severe hydronephrosis.
Longitudinal cohort: changes of infection and morbidity from 2000 to 2014
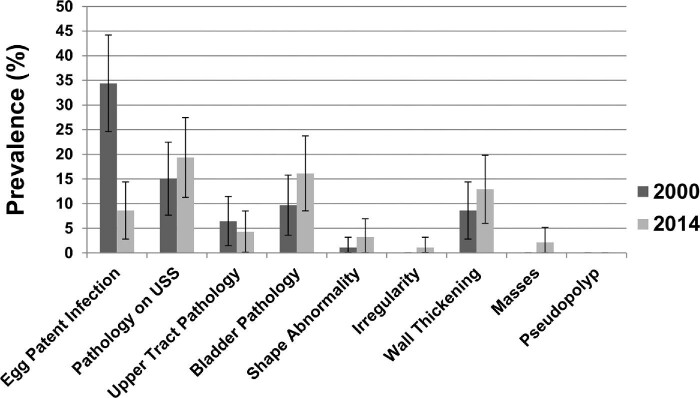
Table 3 and Figures 4 and 5 show the comparative egg-patent infection and pathology rates for the 93 women having examination data in both 2000 and 2014. The rate of egg-patent infection within the longitudinal cohort decreased significantly from 34% (32/93) in 2000 to 9% (8/93) in 2014 (χ2 = 18.3, P < .001), but while not statistically significantly different, the prevalence of urinary tract pathology increased from 15% (14/93) to 19% (18/93). Of the 14 who had been affected in 2000, 11 had cleared their abnormalities, whereas 3 still had abnormal findings—3 had persistent ureteric dilatation, 1 had persistent unilateral hydronephrosis and 1 had persistent bladder wall thickening and a mass. Overall, the rate of upper urinary tract pathology in the longitudinal cohort remained relatively stable, with 6/93 participants in 2000 and 4/93 participants in 2014 showing either ureteric or renal pathology. However, within this cohort the presence of bladder wall thickening had increased by 50% (8/93 in 2000 to 12/93 in 2014 [difference was non-significant]), and while less common, bladder shape abnormality had increased from 1/93 to 3/93, (difference was non-significant).
Table 3.
Individual-level data of egg-patent infection and urinary tract pathology among the cohort of women examined in 2000 and again in 2014.
| 2000 | 2014 | |||
|---|---|---|---|---|
| Characteristics | n | % of total (N=93) (95% CI) | n | % of total (N=93) (95% CI) |
| Egg-patent infection | 32 | 34 (25 to 44) | 8 | 9 (3 to 14) |
| Any urinary tract pathology | 14 | 15 (8 to 22) | 18 | 19 (11 to 28) |
| Upper tract pathology | 6 | 6 (1 to 11) | 4 | 4 (0.1 to 8) |
| Any bladder abnormality | 9 | 10 (4 to 16) | 15 | 16 (8 to 24) |
| Abnormal shape | 1 | 1 (0 to 3) | 3 | 3 (0 to 7) |
| Bladder irregularity | 0 | – | 1 | 1 (0 to 3) |
| Wall thickening | 8 | 9 (3 to 14) | 12 | 13 (6 to 20) |
| Masses | 0 | – | 2 | 2 (0 to 5) |
| Pseudopolyps | 0 | – | 0 | – |
Figure 4.
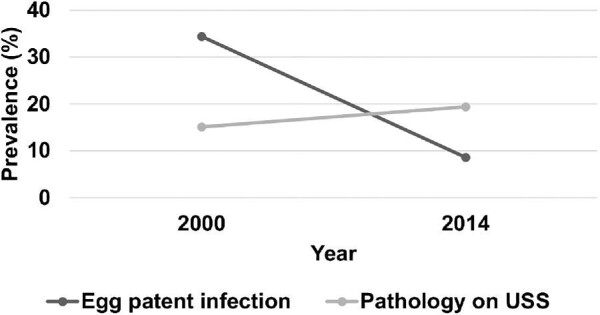
Longitudinal change in the prevalence of egg-patent infection and pathology on ultrasound between 2000 and 2014 (N=93).
Figure 5.
Prevalence of egg-patent infection and urinary tract pathology in a cohort of 93 studied women comparing results in 2000 to those in 2014.
DISCUSSION
To the best of our knowledge, this is the first use of ultrasound assessment for longitudinal follow-up of schistosomiasis pathology over an extended multiyear time period where intermittent distribution of praziquantel has taken place. As expected following implementation of treatment campaigns, the prevalence of active infection was lower than in previous surveys; in the study villages, it had been observed that egg-patent infection among school-age children had fallen from 63.2% in 198428 to 33.2% in 2000–2001.30 While the 6.9% egg-patent infection rate among adult women in 2014 cannot be directly compared with the previous population-level data, it was much lower than the 23% (34/147) infection rate found among untreated adult women in two neighbouring villages in 1986.33 The successful schistosomiasis control programmes in the study area have now included ongoing mass drug administration for school-age children, improved education of schistosomiasis symptoms and prevention in the area, periodic mass drug administration for adults, improved sanitation in the villages and the installation of wells to provide greater access to clean water.34
Bladder wall thickening was the most common form of pathology found on ultrasound, which replicates the findings of earlier ultrasound studies of urogenital schistosomiasis pathology in other endemic areas.35–37 Interestingly, we found more urinary tract pathology than egg-patent infection among adult women in both villages. There are several plausible explanations. First, adults are more likely to have a low egg-excretion infections, in which eggs remain trapped within the bladder wall with fewer eggs shed into the urine, a situation that leads to false negatives as egg exit sites become blocked by fibrosis. In these cases, the active infection is often not detected on urine microscopy of a single urine specimen, as the diagnostic yield is reduced in lower-intensity infections.38
It has also been shown that the excreted egg count in urine does not necessarily correlate with fibrotic pathology among adults35,39,40 and that the likelihood of pathology can be related to an excessive (unmodulated) immune response rather than to the number of excreted eggs per se.41,42 This suggests that in our study's population of adult women there is ongoing morbidity despite no detectable active infection. In endemic areas, older age suggests greater exposure to repeated Schistosoma infection and therefore an increased cumulative exposure to trapped schistosomal eggs. Thus adults present with consolidated anatomical structural damage (as sequelae of their experience with chronic infection) that can be less likely to revert after treatment-related clearance of the parasite.24 In an area of high transmission in Zanzibar, Lyons et al.43 found more urinary tract pathology than active schistosomiasis infection, while in a low-transmission area on the same island the prevalence of urinary tract pathology and active infection were the same. This suggests that the proportion of urinary tract pathology outlasting active infection is more closely related to the aggregate, or cumulative, egg exposures of those specific populations.
The present study included a longitudinal cohort of women having two sequential ultrasound examinations 14 y apart in order to estimate the risk of long-term schistosomiasis-associated urinary tract pathology in an S. haematobium–endemic setting that had received intermittent community-based mass treatments. While active, egg-patent infection rates dropped significantly between 2000 and 2014, the prevalence of urinary tract pathology was marginally increased within our cohort. This suggests that although bladder pathology may have improved in the short term after praziquantel treatment,26 recurrent infections suffered by these women may have caused damage within the bladder wall (leading to abnormal shape and thickening), with further progression during successive infections. Upper urinary tract pathology, while uncommon, remained present in 50% of those who had been found with upper pathology in 2000. This supports our current understanding that ureteric dilatation and hydronephrosis are late-stage changes that do not respond to praziquantel treatment.17,18,20–23,25
Certain limitations of our study are worthy of discussion. Foremost, as our focus was on adult women, children and adult males were not included, which limits the age and gender generalizability of our findings. The process of matching participants to previous data from 2000 limited the lookback cohort size and associated study power. We used a matching process requiring at least two identifying criteria (name, year of birth or household) to ensure that participants were accurately matched with their 2000 data. Many participants were unsure of their exact date of birth or had changed their names, while the villages had expanded greatly in 14 y, making it difficult to track the movement of some households due to relocation. Because of a lack of interval testing, we cannot conclude whether the 2014 pathology seen in this cohort was due to a current, low-intensity S. haematobium infection (potentially not detected on egg microscopy) or whether it was due to exposure during previous infections. In addition, although of potential interest, the multiplicity of a subject's intervening non-study praziquantel treatment frequency could not be independently confirmed. Assessment of urine for current leukocytes, erythrocytes or eosinophils was not done, but could have helped to identify ongoing inflammation of the urinary tract at the time of the 2014 survey. In consideration of diagnostics, future studies should involve more sensitive diagnostic assays (augmenting baseline methods) for better differentiation of active infection in order to distinguish between our two hypotheses. Finally, it remains enigmatic whether schistosomiasis-associated urinary tract pathology does or does not correlate with genital tract manifestations. As public health recognition of the importance of female genital schistosomiasis grows,44 future studies should attempt to integrate urinary and genital morbidity assessments and correlate ultrasound findings with infection markers from both urine and genital samples.
Despite these limitations, our study supports the need for interventions such as mass drug administration, targeted from the age of first exposure to Schistosoma infection, to be repeated at regular intervals to reduce cumulative egg exposure.45 This could prevent the evolution of more advanced chronic disease, which becomes less reversible with treatment.26 In the 2014–2023 interval, national helminth control programs have continued among school-age children and access to primary healthcare has improved within the study area. With a greater understanding of the true disease burden of this neglected tropical disease and by targeting even younger children in mass praziquantel administration to prevent early development of chronic morbidity, we can further strengthen the current efforts to control and eliminate schistosomiasis.
Conclusions
This is the first study to assess the prevalence of schistosomiasis-associated urinary tract pathology following a prolonged 14-y interval. We have found that ultrasound-detected morbidity overshadows egg-patent infection among adult women in this area and therefore the disease burden in this population is greatly underestimated when using only egg microscopy surveillance. The increased availability of portable ultrasound now allows for surveillance of chronic pathology alongside egg microscopy in field studies. Future disease burden estimates should be adjusted accordingly to include the risk of chronic morbidity as well as egg-patent infection.
Supplementary Material
Acknowledgements
We would like to thank the residents of Nganja and Mililani villages, along with Joyce Bongo and Biasha Mohammed Kama, without whom this work would not have been possible.
Contributor Information
Elizabeth Joekes, Department of Radiology, Liverpool University Hospitals NHS Foundation Trust, Liverpool L7 8XP, UK; Department of Clinical Sciences, Liverpool School of Tropical Medicine, Liverpool L3 5QA, UK.
Kate McMonnies, Department of Tropical Disease Biology, Liverpool School of Tropical Medicine, Liverpool L3 5QA, UK.
Andrew Blanshard, Department of Tropical Disease Biology, Liverpool School of Tropical Medicine, Liverpool L3 5QA, UK.
Francis M Mutuku, Department of Environment and Health Science, Technical University of Mombasa, Mombasa, Kenya.
Edmund Ireri, Kenya Medical Research Institute, CCR Radiology Unit, Nairobi, Kenya.
Peter Mungai, Center for Global Health and Diseases, Case Western Reserve University, Cleveland, OH 44106, USA.
J Russell Stothard, Department of Tropical Disease Biology, Liverpool School of Tropical Medicine, Liverpool L3 5QA, UK.
Amaya L Bustinduy, Department of Clinical Research, London School of Hygiene and Tropical Medicine, London WC1E 7HT, UK.
Charles H King, Center for Global Health and Diseases, Case Western Reserve University, Cleveland, OH 44106, USA.
Authors’ contributions
EJ, KM, AB, JRS and ALB conceived the study. EJ, KM, AB, FMM, ALB and CHK designed the study protocol. EJ, KM, EI and AB carried out the clinical assessments. AB, FMM and PM carried out the laboratory testing. EJ, KM, ALB and CHK analysed and interpretated the data. KM drafted the manuscript. EJ, ALB, JRS and CHK critically revised the manuscript for intellectual content. All authors read and approved the final manuscript. EJ, ALB and CHK are guarantors of the paper.
Funding
This research was funded by the Radiology Department of the Royal Liverpool University Hospital, Liverpool, UK. The 2000-era surveys were supported by the National Institutes of Health under grants AI-45473 (National Institute of Allergy and Infectious Diseases) and TW/ES01543 (Fogarty International Center) to CHK.
Competing interests
None declared.
Ethical approval
All participants provided individual written informed consent in accordance with the guidelines of the Declaration of Helsinki. Ethical approval for this study was granted by the Liverpool School of Tropical Medicine, Liverpool, UK (study number 14.014) and Pwani University, Kilifi, Kenya (study reference ERC/MSc/004/2014). All participants had access to translated study information and gave written informed consent before their inclusion. Any information presented here has been anonymized as much as possible. Those participants found to have active schistosomiasis infection during the survey were treated with praziquantel at a dose of 40 mg/kg after the study, as recommended by the WHO. In the 2000-era surveys, the research protocol was approved by the Ethical Review Board of the Kenya Medical Research Institute (KEMRI/RES/7/3/1) and the Institutional Review Board for Human Investigation of University Hospitals of Cleveland (protocol 03-88-34). All subjects found to be infected with S. haematobium were treated with standard doses of praziquantel (40 mg/kg) immediately after the initial morbidity survey.
Data availability
Anonymized individual-level outcomes data are provided in an accompanying Supplementary Table (Supplementary Data file for Joekes et al.xlsx).
References
- 1. World Health Organization . Schistosomiasis and soil-transmitted helminthiases: progress report, 2021. Wkly Epidemiol Rec. 2022;97(48):621–32. [Google Scholar]
- 2. GBD 2016 Disease Injury and Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(4):1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. King CH. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113(2):95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colley DG, Bustinduy AL, Secor WEet al. . Human schistosomiasis. Lancet. 2014;383(9936):2253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bustinduy AL, Randriansolo B, Sturt ASet al. . An update on female and male genital schistosomiasis and a call to integrate efforts to escalate diagnosis, treatment and awareness in endemic and non-endemic settings: the time is now. Adv Parasitol. 2022;115:1–44. [DOI] [PubMed] [Google Scholar]
- 6. Burke ML, Jones MK, Gobert GNet al. . Immunopathogenesis of human schistosomiasis. Parasite Immunol. 2009;31(4):163–76. [DOI] [PubMed] [Google Scholar]
- 7. Wynn TA, Thompson RW, Cheever AWet al. . Immunopathogenesis of schistosomiasis. Immunol Rev. 2004;201(1):156–67. [DOI] [PubMed] [Google Scholar]
- 8. Shebel HM, Elsayes KM, Abou El Atta HMet al. . Genitourinary schistosomiasis: life cycle and radiologic-pathologic findings. Radiographics. 2012;32(4):1031–46. [DOI] [PubMed] [Google Scholar]
- 9. Bonnard P, Boutouaba S, Diakhate Iet al. . Learning curve of vesico-urinary ultrasonography in Schistosoma haematobium infection with WHO practical guide: a “simple to learn” examination. Am J Trop Med Hyg. 2011;85(6):1071–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khalaf I, Shokeir A, Shalaby M.. Urologic complications of genitourinary schistosomiasis. World J Urol. 2012;30(1):31–8. [DOI] [PubMed] [Google Scholar]
- 11. King CH. Lifting the burden of schistosomiasis—defining elements of infection-associated disease and the benefits of antiparasite treatment. J Infect Dis. 2007;196(5):653–5. [DOI] [PubMed] [Google Scholar]
- 12. Hatz CF. The use of ultrasound in schistosomiasis. Adv Parasitol. 2001;48:225–84. [DOI] [PubMed] [Google Scholar]
- 13. Skelly PJ. The use of imaging to detect schistosomes and diagnose schistosomiasis. Parasite Immunol. 2013;35(9–10):295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akpata R, Neumayr A, Holtfreter MCet al. . The WHO ultrasonography protocol for assessing morbidity due to Schistosoma haematobium. Acceptance and evolution over 14 years. Systematic review. Parasitol Res. 2015;114(4):1279–89. [DOI] [PubMed] [Google Scholar]
- 15. Richter J, Hatz C, Campagne Get al. . Ultrasound in schistosomiasis: a practical guide to the standardized use of ultrasonography for the assessment of schistosomiasis-related morbidity. Geneva: World Health Organization; 2000. [Google Scholar]
- 16. Barda B, Coulibaly JT, Hatz Cet al. . Ultrasonographic evaluation of urinary tract morbidity in school-aged and preschool-aged children infected with Schistosoma haematobium and its evolution after praziquantel treatment: a randomized controlled trial. PLoS Negl Trop Dis. 2017;11(2):e0005400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hatz CF, Vennervald BJ, Nkulila Tet al. . Evolution of Schistosoma haematobium-related pathology over 24 months after treatment with praziquantel among school children in southeastern Tanzania. Am J Trop Med Hyg. 1998;59(5):775–81. [DOI] [PubMed] [Google Scholar]
- 18. Wagatsuma Y, Aryeetey ME, Sack DAet al. . Resolution and resurgence of Schistosoma haematobium-induced pathology after community-based chemotherapy in Ghana, as detected by ultrasound. J Infect Dis. 1999;179(6):1515–22. [DOI] [PubMed] [Google Scholar]
- 19. Tamarozzi F, Ursini T, Ronzoni Net al. . Prospective cohort study using ultrasonography of Schistosoma haematobium-infected migrants. J Travel Med. 2021;28(6):taab122. [DOI] [PubMed] [Google Scholar]
- 20. King CH, Muchiri EM, Ouma JH.. Age-targeted chemotherapy for control of urinary schistosomiasis in endemic populations. Mem Inst Oswaldo Cruz. 1992;87(Suppl 4):203–10. [DOI] [PubMed] [Google Scholar]
- 21. Strahan R, McAdam D, Schneider ME.. Sonographic response in the liver and urinary bladder of children 14 months after treatment for schistosomiasis. Trop Doct. 2013;43(2):71–4. [DOI] [PubMed] [Google Scholar]
- 22. Subramanian AK, Mungai P, Ouma JHet al. . Long-term suppression of adult bladder morbidity and severe hydronephrosis following selective population chemotherapy for Schistosoma haematobium. Am J Trop Med Hyg. 1999;61(3):476–81. [DOI] [PubMed] [Google Scholar]
- 23. Carlton EJ, Hsiang M, Zhang Yet al. . The impact of Schistosoma japonicum infection and treatment on ultrasound-detectable morbidity: a five-year cohort study in southwest China. PLoS Negl Trop Dis. 2010;4(5):e685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Magak P, Chang-Cojulun A, Kadzo Het al. . Case-control study of posttreatment regression of urinary tract morbidity among adults in Schistosoma haematobium-endemic communities in Kwale County, Kenya. Am J Trop Med Hyg. 2015;93(2):371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ouma JH, King CH, Muchiri EMet al. . Late benefits 10–18 years after drug therapy for infection with Schistosoma haematobium in Kwale District, Coast Province, Kenya. Am J Trop Med Hyg. 2005;73(2):359–64. [PubMed] [Google Scholar]
- 26. Andrade G, Bertsch DJ, Gazzinelli Aet al. . Decline in infection-related morbidities following drug-mediated reductions in the intensity of Schistosoma infection: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2017;11(2):e0005372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muchiri EM, Ouma JH, King CH.. Dynamics and control of Schistosoma haematobium transmission in Kenya: an overview of the Msambweni Project. Am J Trop Med Hyg. 1996;55(5 Suppl):127–34. [DOI] [PubMed] [Google Scholar]
- 28. Satayathum SA, Muchiri EM, Ouma JHet al. . Factors affecting infection or reinfection with Schistosoma haematobium in coastal Kenya: survival analysis during a nine-year, school-based treatment program. Am J Trop Med Hyg. 2006;75(1):83–92. [PMC free article] [PubMed] [Google Scholar]
- 29. Richter J, Wagatsuma Y, Aryeetey Met al. . Sonographic screening for urinary tract abnormalities in patients with Schistosoma haematobium infection: pitfalls in examining pregnant women. Bull World Health Org. 1996;74(2):217–21. [PMC free article] [PubMed] [Google Scholar]
- 30. King CH, Blanton RE, Muchiri EMet al. . Low heritable component of risk for infection intensity and infection-associated disease in urinary schistosomiasis among Wadigo village populations in Coast Province, Kenya. Am J Trop Med Hyg. 2004;70(1):57–62. [PubMed] [Google Scholar]
- 31. Doehring E, Vester U, Ehrich JHHet al. . Circadian variation of ova excretion, proteinuria, haematuria, and leukocyturia in urinary schistosomiasis. Kidney Int. 1985;27(4):667–71. [DOI] [PubMed] [Google Scholar]
- 32. World Health Organization . Basic laboratory methods in medical parasitology. Geneva: World Health Organization; 1991. [Google Scholar]
- 33. Hodder SL, Mahmoud AAF, Sorenson Ket al. . Predisposition to urinary tract epithelial metaplasia in Schistosoma haematobium infection. Am J Trop Med Hyg. 2000;63(3):133–8. [DOI] [PubMed] [Google Scholar]
- 34. el Kholy H, Arap Siongok TK, Koech Det al. . Effects of borehole wells on water utilization in Schistosoma haematobium endemic communities in Coast Province, Kenya. Am J Trop Med Hyg. 1989;41(2):212–9. [DOI] [PubMed] [Google Scholar]
- 35. Elmadani AE, Hamdoun AO, Monis Aet al. . Ultrasound findings in urinary schistosomiasis infection in school children in the Gezira State Central Sudan. Saudi J Kidney Dis Transpl. 2013;24(1):162–7. [DOI] [PubMed] [Google Scholar]
- 36. King CH. Ultrasound monitoring of structural urinary tract disease in S. haematobium infection. Mem Inst Oswaldo Cruz. 2002;97(Suppl 1):149–52. [DOI] [PubMed] [Google Scholar]
- 37. Ma'aji SM, Adamu B.. Pattern of urinary bladder sonographic findings in patients evaluated for urinary schistosomiasis. West Afr J Radiol. 2015;22(2):92–96. [Google Scholar]
- 38. Vinkeles Melchers NV, van Dam GJ, Shaproski Det al. . Diagnostic performance of Schistosoma real-time PCR in urine samples from Kenyan children infected with Schistosoma haematobium: day-to-day variation and follow-up after praziquantel treatment. PLoS Negl Trop Dis. 2014;8(4):e2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brouwer KC, Ndhlovu PD, Wagatsuma Yet al. . Epidemiological assessment of Schistosoma haematobium-induced kidney and bladder pathology in rural Zimbabwe. Acta Trop. 2003;85(3):339–47. [DOI] [PubMed] [Google Scholar]
- 40. King CH, Keating CE, Muruka JFet al. . Urinary tract morbidity in schistosomiasis haematobia: associations with age and intensity of infection in an endemic area of Coast Province, Kenya. Am J Trop Med Hyg. 1988;39(4):361–8. [DOI] [PubMed] [Google Scholar]
- 41. Mutapi F. Improving diagnosis of urogenital schistosome infection. Expert Rev Anti Infect Ther. 2011;9(10):863–5. [DOI] [PubMed] [Google Scholar]
- 42. Wamachi AN, Mayadev JS, Mungai PLet al. . Increased ratio of tumor necrosis factor-alpha to interleukin-10 production is associated with Schistosoma haematobium-induced urinary-tract morbidity. J Infect Dis. 2004;190(11):2020–30. [DOI] [PubMed] [Google Scholar]
- 43. Lyons B, Stothard R, Rollinson Det al. . A comparison of urinary tract pathology and morbidity in adult populations from endemic and non-endemic zones for urinary schistosomiasis on Unguja Island, Zanzibar. BMC Infect Dis. 2009;9:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Engels D, Hotez PJ, Ducker Cet al. . Integration of prevention and control measures for female genital schistosomiasis, HIV and cervical cancer. Bull World Health Org. 2020;98(9):615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stothard JR, Sousa-Figueiredo JC, Betson Met al. . Schistosomiasis in African infants and preschool children: let them now be treated! Trends Parasitol. 2013;29(4):197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized individual-level outcomes data are provided in an accompanying Supplementary Table (Supplementary Data file for Joekes et al.xlsx).