In the current issue of Circulation: Arrhythmia and Electrophysiology, Berman et al1 publish a new analysis on the cost-effectiveness of catheter ablation for atrial fibrillation (AF). Their article opens a new chapter in the story about the health economic value of AF ablation by, for the first time, explicitly considering the impact of ablation on the tendency for AF to progress from a paroxysmal to a persistent pattern.
See Article by Berman et al
The study from Berman et al involved the creation of a “time-heterogenous individual-level state-transition” model intended to simulate and then project, with a lifetime perspective, the likely outcomes of patients treated in the ATTEST trial (The Randomized Controlled Atrial Fibrillation Progression Trial).2 ATTEST randomized 255 patients with paroxysmal AF refractory to 1 to 2 antiarrhythmic or rate control drugs (51% Vaughan-Williams Class I or III drugs at baseline) to radiofrequency-based AF ablation or treatment with continued or alternative drug therapy (42% new Class I or III agents in the drug arm). ATTEST reported a 3-year Kaplan-Meier rate of first time persistent AF or atrial tachycardia of 2.4% with ablation, versus 17.4% with drug therapy.
In the health economic model developed by Berman et al, simulated patients could occupy one of the 2 main health states: “early AF,” during which patients could stay in sinus rhythm or have recurrences of paroxysmal AF, or “advanced AF” marked by persistent AF/atrial tachycardia and its consequences. In the model, the progression from early to advanced AF was unidirectional, with the probability of this transition over time projected based on the trial data. Costs and quality of life were not measured in ATTEST. The authors therefore obtained estimates of these and other crucial model inputs from external literature. In the model, patients treated initially with drugs crossed over to ablation at a rate of 12% per year. The disease simulation model predicted a small (0.09) gain in lifetime quality-adjusted life years (QALYs) with ablation and roughly neutral lifetime costs (point estimate of $10 cost savings [95% CI, −$2600 to 2300]), leading to the conclusion that ablation was economically “dominant” (less expensive and more effective) long term in this population.
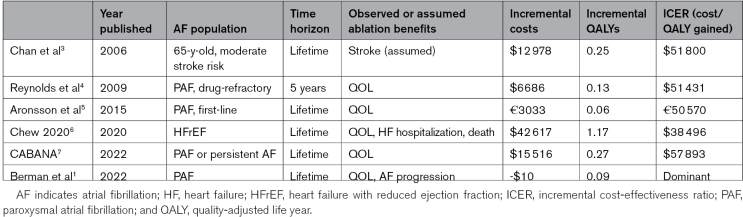
A number of previous studies have attempted to estimate the cost-effectiveness of AF ablation in different patient groups and clinical contexts. Results from selected, mainly US-based studies are shown in the Table. Though these studies appear to be similar on the surface, they vary widely in patient characteristics (AF pattern, presence of absence of heart failure), timing of ablation (prior to or after failure of antiarrhythmic drugs), comparator (mainly antiarrhythmic drugs), and analytic assumptions (time horizon, patient outcomes, amount of crossover between groups, etc). Despite these differences, prior cost-effectiveness models of AF ablation have produced concordant bottom-line results: in all cases ablation increased QALYs, usually by small amounts (larger in heart failure, where a survival advantage has been reported8), increased overall costs, and appeared to provide high economic value,9 with incremental cost-effectiveness ratios in the range of around $40 000 to 60 000 per QALY gained.
Table.
Selected Cost-Effectiveness Analysis of AF Ablation
In the earliest cost-effectiveness studies of AF ablation, the clinical benefits of AF ablation, for example, to reduce the risk of stroke, were hypothetical.3 Later studies anchored QALY gains on measured improvements in quality of life,4,5,7 now well established,10 or on improved heart failure outcomes as documented in randomized trials.6 Although some prior studies accounted for potential treatment failure, none directly factored in progression of the AF pattern.
What explains the more favorable findings of the current study? Part of the answer is likely the high rate of crossover from drugs to ablation assumed in the model, as was observed in ATTEST.2 When the crossover rate is high, a large proportion of each group ultimately winds up with the same therapy, and long-term costs and outcomes tend to converge, making both incremental costs and QALYs small. A similar phenomenon was seen in an earlier cost-effectiveness model based on the MANTRA-PAF study (Medical Antiarrhythmic Treatment or Radiofrequency Ablation in Paroxysmal Atrial Fibrillation).5 When the authors instead assumed a lower rate of crossover as observed in the CABANA trial (Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation; 28% at 5 years), ablation was associated with an $1800 increase in lifetime costs and an incremental cost-effectiveness ratio of $22 700 per QALY gained.1
There are a few other reasons to be cautious about the conclusions of this study. Most health economic models, like this one, rely on simplifying assumptions about the disease state under study, as well as external literature for inputs not measured in clinical studies. A health economic analysis like the one recently published by the CABANA investigators7 is less commonly achievable—as detailed data collection over long follow-up requires substantial planning and investment—but more grounded in empirical evidence. The current study importantly assumes that once patients develop persistent AF/atrial tachycardia, there is no going back with respect to increased long-term resource utilization and risk of adverse events. Yet, many patients achieve very good results after ablation for persistent AF, especially when it is not long-standing. The model, however, treats all forms of persistent AF, whether recent, long-standing, or permanent, the same.
The model also relies heavily on differential rates of resource utilization and costs once patients progress into the persistent AF state, derived primarily from a recent study examining outcomes after ablation for paroxysmal and persistent AF from a large US insurance claims database.11 The potential problem with this approach is that there are many observable (and unobservable) differences in age, underlying heart disease and comorbidity between patients with persistent and paroxysmal AF, such that the source data for the assumed cost differences between these groups likely suffered from confounding, making the link between the claims analysis and the ATTEST population tenuous.
It should also be remembered that ATTEST studied paroxysmal AF patients who in most cases had already failed at least 1 antiarrhythmic drug. The superiority of ablation over antiarrhythmic drugs in this circumstance was established by multiple randomized trials some time ago, which may help explain why ATTEST terminated early due to slow enrollment.2 More recently, extended follow-up of the EARLY-AF trial (Early Aggressive Invasive Intervention for Atrial Fibrillation)12 showed that AF ablation (with the cryoballoon system) also reduced progression from paroxysmal to persistent AF in a first-line rhythm control setting. The difference in progression observed in EARLY-AF was smaller (1.9% versus 7.4% at 3 years) than in ATTEST, perhaps related to the interventions occurring more upstream.
Given the methodological constraints and source data limitations, my opinion is that the precise estimates reported by Berman et al should be regarded as speculative. Nonetheless, I readily accept the premise that avoiding the progression from paroxysmal to persistent AF can only be good for patients and should only enhance the value of AF ablation. It is just difficult to determine by exactly how much.
In addition to the recent EAST-AFNET 4 study (The Early Treatment of Atrial Fibrillation for Stroke Prevention Trial),13 which showed that early rhythm control in general provides benefits to patients with AF, we now have evidence from 2 randomized trials that AF ablation specifically reduces the likelihood of AF progression compared with drug therapy.2,12 In conclusion, new and emerging data support the view that AF ablation, previously found to be a high value therapy by multiple investigative groups, adds further value when its ability to reduce progression from paroxysmal to persistent AF is considered: addition by subtraction.
Article Information
Disclosures
Dr Reynolds reports consulting relationships with Medtronic, Sanofi, and iRhythm and serves on a data safety and monitoring board for Affera.
Footnotes
For Sources of Funding and Disclosures, see page 190.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
References
- 1.Berman AE, Kabiri M, Wei T, Galvain T, Sha Q, Kuck K-H. Economic and health value of delaying atrial fibrillation progression using radiofrequency cathter ablation. Circ Arrhythm Electrophysiol. 2022;16:e011237. doi: 10.1161/CIRCEP.122.011237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuck KH, Lebedev DS, Mikhaylov EN, Romanov A, Geller L, Kalejs O, Neumann T, Davtyan K, On YK, Popov S, et al. Catheter ablation or medical therapy to delay progression of atrial fibrillation: the randomized controlled atrial fibrillation progression trial (ATTEST). Europace. 2021;23:362–369. doi: 10.1093/europace/euaa298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan PS, Vijan S, Morady F, Oral H. Cost-effectiveness of radiofrequency catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2006;47:2513–2520. doi: 10.1016/j.jacc.2006.01.070 [DOI] [PubMed] [Google Scholar]
- 4.Reynolds MR, Zimetbaum P, Josephson ME, Ellis E, Danilov T, Cohen DJ. Cost-effectiveness of radiofrequency catheter ablation compared with antiarrhythmic drug therapy for paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:362–369. doi: 10.1161/CIRCEP.108.837294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aronsson M, Walfridsson H, Janzon M, Walfridsson U, Nielsen JC, Hansen PS, Johannessen A, Raatikainen P, Hindricks G, Kongstad O, et al. The cost-effectiveness of radiofrequency catheter ablation as first-line treatment for paroxysmal atrial fibrillation: results from a MANTRA-PAF substudy. Europace. 2015;17:48–55. doi: 10.1093/europace/euu188 [DOI] [PubMed] [Google Scholar]
- 6.Chew DS, Loring Z, Anand J, Fudim M, Lowenstern A, Rymer JA, Weimer KED, Atwater BD, DeVore AD, Exner DV, et al. Economic evaluation of catheter ablation of atrial fibrillation in patients with heart failure with reduced ejection fraction. Circ Cardiovasc Qual Outcomes. 2020;13:e007094. doi: 10.1161/CIRCOUTCOMES.120.007094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chew DS, Li Y, Cowper PA, Anstrom KJ, Piccini JP, Poole JE, Daniels MR, Monahan KH, Davidson-Ray L, Bahnson TD, et al. ; CABANA Investigators. Cost-effectiveness of catheter ablation versus antiarrhythmic drug therapy in atrial fibrillation: the CABANA randomized clinical trial. Circulation. 2022;146:535–547. doi: 10.1161/CIRCULATIONAHA.122.058575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, et al. ; CASTLE-AF Investigators. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. doi: 10.1056/NEJMoa1707855 [DOI] [PubMed] [Google Scholar]
- 9.Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, et al. ; ACC/AHA Task Force on Performance Measures. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129:2329–2345. doi: 10.1161/CIR.0000000000000042 [DOI] [PubMed] [Google Scholar]
- 10.Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, Daniels MR, Bahnson TD, Poole JE, Rosenberg Y, et al. ; CABANA Investigators. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1275–1285. doi: 10.1001/jama.2019.0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman DJ, Field ME, Rahman M, Goldstein L, Sha Q, Sidharth M, Khanna R, Piccini JP. Catheter ablation and healthcare utilization and cost among patients with paroxysmal versus persistent atrial fibrillation. Heart Rhythm O2. 2021;2:28–36. doi: 10.1016/j.hroo.2020.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrade JG, Deyell MW, Macle L, Wells GA, Bennett M, Essebag V, Champagne J, Roux JF, Yung D, Skanes A, et al. ; EARLY-AF Investigators. Progression of atrial fibrillation after cryoablation or drug therapy. N Engl J Med. 2023;388:105–116. doi: 10.1056/NEJMoa2212540 [DOI] [PubMed] [Google Scholar]
- 13.Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, et al. ; EAST-AFNET 4 Trial Investigators. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–1316. doi: 10.1056/NEJMoa2019422 [DOI] [PubMed] [Google Scholar]