Abstract
The critical role of alpha-synuclein in Parkinson’s disease represents a pivotal discovery. Some progress has been made over recent years in identifying disease-modifying therapies for Parkinson’s disease that target alpha-synuclein. However, these treatments have not yet shown clear efficacy in slowing the progression of this disease. Several explanations exist for this issue. The pathogenesis of Parkinson’s disease is complex and not yet fully clarified and the heterogeneity of the disease, with diverse genetic susceptibility and risk factors and different clinical courses, adds further complexity. Thus, a deep understanding of alpha-synuclein physiological and pathophysiological functions is crucial.
In this review, we first describe the cellular and animal models developed over recent years to study the physiological and pathological roles of this protein, including transgenic techniques, use of viral vectors and intracerebral injections of alpha-synuclein fibrils. We then provide evidence that these tools are crucial for modelling Parkinson’s disease pathogenesis, causing protein misfolding and aggregation, synaptic dysfunction, brain plasticity impairment and cell-to-cell spreading of alpha-synuclein species. In particular, we focus on the possibility of dissecting the pre- and postsynaptic effects of alpha-synuclein in both physiological and pathological conditions. Finally, we show how vulnerability of specific neuronal cell types may facilitate systemic dysfunctions leading to multiple network alterations.
These functional alterations underlie diverse motor and non-motor manifestations of Parkinson’s disease that occur before overt neurodegeneration. However, we now understand that therapeutic targeting of alpha-synuclein in Parkinson’s disease patients requires caution, since this protein exerts important physiological synaptic functions. Moreover, the interactions of alpha-synuclein with other molecules may induce synergistic detrimental effects. Thus, targeting only alpha-synuclein might not be enough. Combined therapies should be considered in the future.
Keywords: alpha-synuclein, Parkinson’s disease, synaptic plasticity, striatum, dopamine
Calabresi et al. review the physiological and pathological roles of alpha-synuclein, focusing on its pre- and post-synaptic effects. Discussing findings from preclinical models of Parkinson’s disease, they explore how alterations at the cellular level contribute to the spread of pathology at the network level.
Introduction
Mutations in the gene that codifies alpha-synuclein (α-syn) cause Parkinson’s disease (PD).1 This discovery of a genetic defect leading to PD opened new avenues for investigating the molecular basis of the disorder,2 and it is now clear that this protein also plays a critical role in the sporadic forms of PD.
Some progress has been made over recent years in identifying disease-modifying therapies for PD that target α-syn.3 However, these treatments have not yet fully met the required end point, including a disease-modifying effect.4-6 Several explanations exist for this failure. First, the pathogenesis of PD is complex and not yet fully clarified. Thus, the ideal target and time window for treatment are unknown. Second, the heterogeneity of the disease, with diverse genetic susceptibility and risk factors and different clinical courses, adds further complexity.
Another obstacle to an effective α-syn-related disease-modifying therapy might be the lack of specificity of these approaches in distinguishing between the detrimental effects of the protein and its physiological functions. The need to dissect these aspects is emerging, along with the concept of proteinopenia versus proteinopathy, concerning the role of α-syn in PD.7 In this review, we explain how these roles are intermingled at the synaptic level. Although the physiological function of α-syn is yet to be fully elucidated, this protein is enriched in presynaptic compartments, where it can associate with vesicles and membranes.8 The synapse is also the scenario in which pathological α-syn exerts its early detrimental effects, preventing vesicle clustering and altering postsynaptic responses to transmitters, which in turn cause impairment of synaptic plasticity.9 This cascade of early abnormal events might be responsible for a network dysfunction before the occurrence of overt neurodegeneration.
We will evaluate new advances in the understanding of synaptic changes modulated by α-syn that occur before neuronal death and how they influence synaptic plasticity in the basal ganglia and other brain networks. Moreover, we will discuss how and why these new findings support the idea that any new therapeutic approaches should target synaptopathy and indicate that future studies across multiple neuron types and circuits are mandatory.
New models to investigate synaptopathy
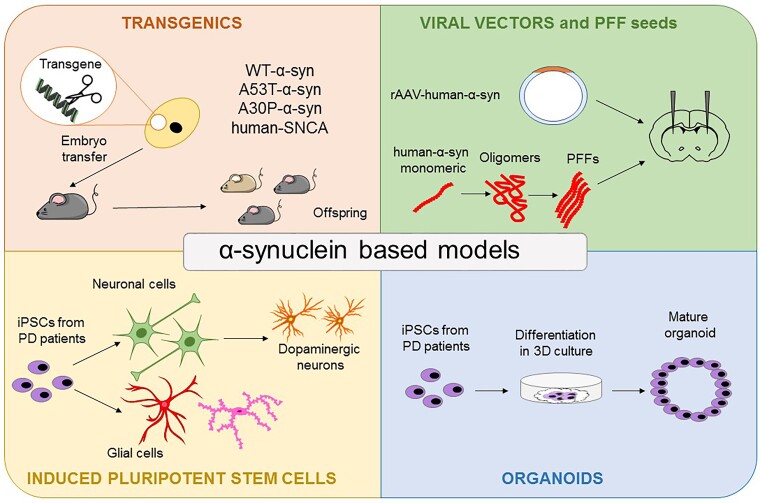
Animal PD models are critical in investigating early α-syn-induced synaptic and plastic dysfunctions. Invertebrate PD models are particularly useful for high throughput-screening applications, whereas mammalian models are needed to explore complex motor and non-motor features.10-12 In these animal models, nigrostriatal dopaminergic dysfunction can be induced via overexpression of α-syn using viral vectors or transgenic techniques.13 In addition, protein aggregation pathology can be triggered by inoculating α-syn pre-formed fibrils (PFF) in the substantia nigra or the striatum of rodents. This new generation of animal models of PD, based on ectopic expression, overexpression or intracerebral injection of the protein α-syn, slowly and gradually develop inclusions of aggregated α-syn and α-syn-mediated neuronal loss, replicating the pathological hallmarks of PD (Fig. 1). Using these models, scientists have pinpointed not only the effects of toxic forms of α-syn leading to neurodegeneration, but also their role in processes that occur before cell death, such as changes in synaptic transmission, basal ganglia plasticity and mechanisms underlying motor learning.14-16
Figure 1.
α-Syn-based experimental models. The top left panel shows genome-editing techniques that allow for the creation of transgenic parkinsonian animals carrying specific α-syn mutations. The top right panel represents animal models obtained either by brain inoculation of adeno-associated viral vectors (AAV) carrying mutant α-syn or of α-syn preformed fibrils (PFFs). The bottom left panel shows in vitro models using induced pluripotent stem cells (iPSCs) derived from patients with Parkinson's disease (PD) to obtain dopaminergic neurons or glial cells. In the bottom right panel, the generation of 3D organoids is shown. WT = wild-type.
Another example of the successful application of neurophysiology in the investigation of α-syn is the use of induced pluripotent stem cell (iPSC) technology for the differentiation and growth of human dopaminergic neurons in vitro17,18 (Fig. 1). IPSCs derived from PD patients carrying the α-syn triplication mutation can be converted into dopaminergic neurons to study their firing activity.19 In human-like dopaminergic neurons, α-syn overexpression dysregulates firing activity, abolishing pace-maker activity and inducing abnormal bursting discharge.19 This effect is caused by reduced functional availability of D2 receptors, resulting in altered dopamine (DA) release and neuronal morphology. Notably, the D2 receptor agonist quinpirole can restore the altered firing activity of patient-derived dopaminergic neurons to normal levels. These results provide novel insights into the pre-degenerative neurophysiological alterations induced by α-syn overexpression. Several studies are now using iPSC-derived organoids from PD patients to investigate cell physiology in a more integrative manner18 (Fig. 1). This technology might represent a more physiological platform for mimicking cell-cell and cell-matrix interactions than iPSC cultures. However, to reproduce the complexity of the brain’s environment, particularly of synaptic connections, researchers recognize the need to further develop and refine these iPSC-based tools. In human iPSC-based models, it has also been shown that α-syn mutations induce protein aggregates and early axonal dysfunction between synaptically linked neurons.20,21
The effects of extracellular α-syn have also recently been investigated using both primary neuronal cultures and in vivo microdialysis.22 While it is now clear that α-syn can affect the firing discharge of specific neuronal populations, recent findings suggest that neuronal activity modulates the release of α-syn and might regulate the spread of α-syn pathology. Indeed, the relationship between α-syn and neuronal activity is now considered bidirectional.22 More specifically, the physiological release of endogenous α-syn depends highly on intrinsic neuronal activities. Accordingly, with the increase of neuronal activity, the release of this protein rapidly increases, while when the activity is blocked, it decreases. Also, in vivo microdialysis experiments in freely moving wild-type mice revealed that a large proportion of extracellular α-syn originates from neuronal activity-dependent pathways. Antagonists of glutamate ionotropic receptors reduce extracellular α-syn levels, suggesting that this excitatory system plays a key role in the activity-dependent release of α-syn. These experiments suggest that dysregulation of excitatory pathways might trigger extracellular release of α-syn and possibly its trans-synaptic propagation. More recently, in line with these findings, it has been shown that α-syn-mediated neuronal degeneration can be enhanced in organotypic brain slice cultures by increasing extracellular potassium or applying GABAA antagonist or a glutamate receptor agonist.23 In support of the hypothesis that hyperexcitability in dopaminergic neurons might increase vulnerability and produce behavioural abnormalities, in vivo experiments revealed that chronic hyperexcitability induced by a chemogenetic approach in an α-syn-based rat model causes both motor alterations and α-syn pathology in the absence of neuronal death. These effects occur via an enhanced neuronal discharge, since these alterations are corrected by reducing neuronal activity.24
All these different experimental approaches strongly indicate that synaptic impairments and axonal degeneration precede neuronal loss. Thus, α-syn-induced early synaptic changes could be a target to prevent disease onset and slow progression. Interestingly, imaging of PD patients with radioligands, post-mortem studies and α-syn-related animal models of PD demonstrate abnormalities in presynaptic terminals and postsynaptic dendritic spines of striatal neurons.25
Presynaptic mechanisms
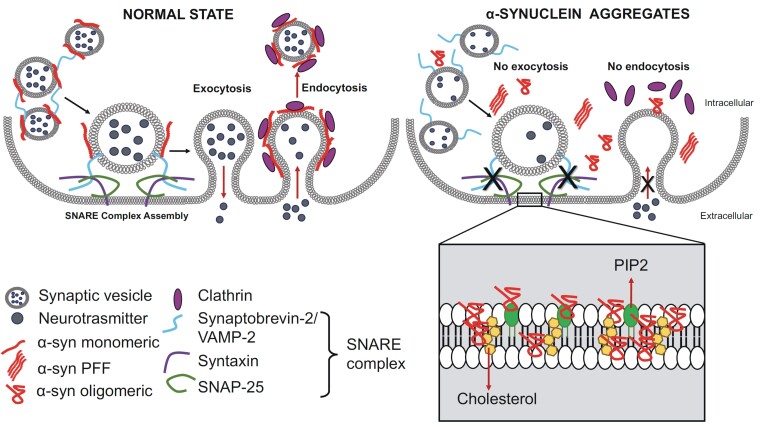
Recent experimental findings have shown that altered forms of α-syn cause a loss-of-function of synaptic transmission by altering the role of this protein at the presynaptic level. In fact, α-syn localizes at presynaptic terminals, where it interacts with membranes, and its overexpression and aggregation slow vesicle exocytosis. Also, the C-terminal calcium-binding site of α-syn exerts an essential role in modulating vesicle interaction26,27 (Fig. 2).
Figure 2.
Physiological and pathological functions of α-syn in vesicle trafficking and its interaction with membrane lipids. The left panel shows the physiological condition in which α-syn monomers control exocytosis by interacting with synaptic vesicle proteins such as VAMP2/synaptobrevin-2, syntaxin and SNAP-25, promoting SNARE complex formation to control the exocytosis process. Monomeric α-syn also controls endocytosis through functional interactions with clathrin. The right panel describes the pathological state in which the accumulation of α-syn aggregates [oligomers and preformed fibrils (PFF)] interferes with presynaptic function. Under this condition, SNARE-mediated vesicle fusion to the plasma membrane is inhibited, leading to abnormal control of neurotransmitter release and transmembrane trafficking. α-Syn aggregates also arrest clathrin-mediated endocytosis. The inset shows how α-syn oligomers interact with membrane lipids, cholesterol and phosphatidylinositol 4,5-bisphosphate (PIP2), altering synaptic membrane function and integrity.
Through its membrane-bound state, α-syn modulates neurotransmitter release by controlling several processes, including vesicle clustering and docking and homeostasis of synaptic vesicle pools.28 It interacts with the synaptic vesicle SNARE protein VAMP2/synaptobrevin-2, the synaptic vesicle-attached synapsins and the synaptic vesicle membrane itself. In line with its physiological functions, abnormal and mutant α-syn impair vesicle trafficking and alter lipid homeostasis.29-31 In particular, α-syn has been implicated in regulating neuronal cholesterol in lipid rafts, which are necessary for synaptic localization, vesicle cycling and modulation of synaptic integrity. On the other hand, cholesterol facilitates interactions between α-syn oligomers.32 The interaction of α-syn with the acidic phosphoinositides has recently been investigated in studies showing that α-syn colocalizes with phosphatidylinositol 4,5-bisphosphate and the phosphorylated active form of the clathrin adaptor protein 2. Interestingly, mutations in α-syn alter clathrin-mediated endocytosis in central neurons33 (Fig. 2).
In a Drosophila model, the combined use of histological, biochemical, behavioural and electrophysiological techniques has shown that overexpression of human α-syn causes accumulation of this protein in terminals, altering the presynaptic active zone.34 These α-syn-mediated presynaptic changes cause impaired neuronal function and behavioural deficits before the progressive degeneration of dopaminergic neurons. Similar abnormalities in presynaptic active zone proteins are observed in brain samples of patients with synucleinopathies, suggesting that presynaptic accumulation of α-syn impairs the active zone and causes early neuronal dysfunction.34
The physiological role of α-syn in presynaptic terminals and in the control of DA release has also recently been investigated in vivo in rodents.35 α-Syn is responsible for facilitating DA release triggered by action potential bursts separated by short intervals (seconds) and depression of release after longer intervals between bursts (minutes). These forms of presynaptic plasticity are in line with a role of α-syn in the enhancement of synaptic vesicle fusion and turnover. Moreover, these findings further support that the presynaptic effects of α-syn depend on specific patterns of neuronal activity and that the interaction between the release of this protein and neuronal activity is bidirectional. Thus, we can speculate that pathological α-syn, by affecting neuronal discharge, influences its own release, creating a dangerous and vicious cycle.
At this point, the critical question is why presynaptic mechanisms are dysfunctional and produce impairments that take place much earlier than neuronal death. Two recent studies have addressed this question, suggesting that nigrostriatal synaptic mitochondrial energy metabolism plays a critical role. In the first study, a genetic approach was used to selectively disrupt mitochondrial complex I in mouse dopaminergic neurons to mimic a metabolic deficit that has been observed consistently in PD patients.36 Although this change in metabolism did not compromise the survival of dopaminergic neurons, it progressively disrupted dopaminergic cell functioning by reducing DA release in the dorsolateral striatum. Deficits in motor learning and subtle motor deficit were also coupled with this axonal dysfunction. Thus, the dysfunction of mitochondrial complex I alone is sufficient to impair DA release and cause early human-like parkinsonism. Moreover, a direct interaction between α-syn and mitochondrial complex I has been established. In particular, α-syn overexpression can disrupt complex I integrity, necessary for the control of neuronal mitochondrial morphology and functioning.37
In the second study, a rat model overexpressing the human mutated A53T α-syn in the substantia nigra pars compacta (SNpc) was used.38 The temporal sequence of functional and structural changes at striatal synapses was analysed before the appearance of evident parkinsonian signs. Sequential window acquisition by mass spectrometry techniques identified that proteins involved in energy metabolism were deregulated. Interestingly, dysfunctional mitochondrial bioenergetics was followed by a decrease in the number of DA terminals and morphological and ultrastructural alterations. Electron microscopy revealed an abnormal accumulation of autophagic and endocytic vesicles within the spared dopaminergic fibres. Ultrastructural signs of aberrant plasticity within glutamatergic synapses, such as a reduction in axo-spinous synapses and an increase in perforated postsynaptic densities, were observed. This evidence strongly supports the hypothesis that synaptic energy failure caused by dysfunctional mitochondrial activity at nigrostriatal terminals represents an early event in the disease course.
The issue of a causative relationship among bioenergetic demand, axonal arbor size and vulnerability of highly branched dopaminergic nigrostriatal axons has been widely discussed.39 Synaptic transmission is a key determinant of cellular energy use, and neurons with multiple active axon terminals along with a highly branched axonal arborization, such as nigral dopaminergic neurons, require a massive energy supply. Moreover, SNpc dopaminergic neurons also demonstrated the highest rates of basal superoxide production, which supports the notion that elevated mitochondrial oxidative phosphorylation might be the origin of chronically elevated oxidative stress in these neurons that leads to presynaptic dysfunction.39
The idea that abnormalities in mitochondrial function are a critical step in initiating dopaminergic dysfunction, leading to parkinsonian phenotypes and ultimately to Lewy body (LB) pathology, is also supported by the pathophysiology of genetic forms of PD, such as those caused by LRRK2 mutations.40
Postsynaptic mechanisms
While the presynaptic functions of α-syn have been investigated for decades, its postsynaptic effects have been much less explored. Nevertheless, it has recently been shown that altered levels and forms of α-syn, acting at the postsynaptic level, perturb homeostatic and synaptic functions through various mechanisms9 (Fig. 3). In particular, in vivo and in vitro studies show an early impact of α-syn on glutamatergic neurotransmission. Indeed, evidence supports the role of α-syn in modulating glutamatergic ionotropic and metabotropic receptor activity through interaction with other proteins. Also, post-translational modifications may play a role. Finally, α-syn seems to have an impact on the glutamatergic activity of astrocytes.
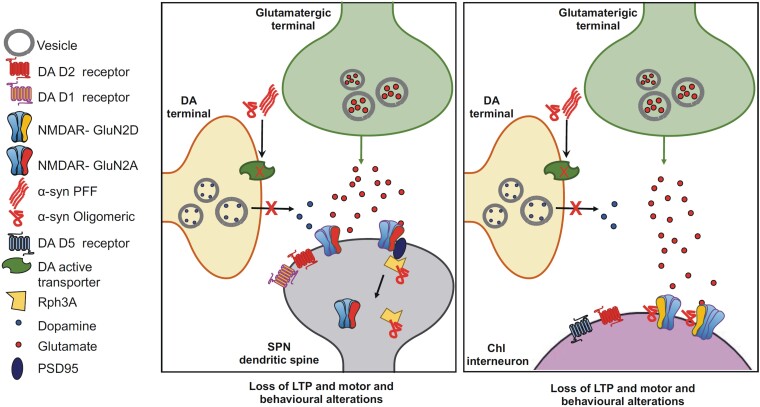
Figure 3.
Schematic representation of α-syn-mediated synaptic alterations in striatal SPNs and ChIs in models of early PD. The left panel shows how extracellular α-syn aggregates cause reduced striatal DAT levels and dopamine release from the dopaminergic terminal. α-Syn oligomers perturb the functional interaction between rabphilin 3A (Rph3A) and the GluN2A-expressing NMDAR in the postsynaptic density of spiny projection neurons (SPN). The right panel represents how α-syn oligomers interact with the NMDAR-expressing GluN2D subunit in the striatal cholinergic interneurons (ChI). These synaptic changes block the induction of long-term potentiation (LTP) in both neuronal types (SPNs and ChIs), causing motor and behavioural alterations. DA = dopamine; PFF = preformed finril.
Aggregated α-syn seems to alter the subunit composition and function of N-methyl-D-aspartate receptors (NMDAR) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR). In a study using post-mortem human brain tissue, levels of α-syn monomers and oligomers were found to increase with age selectively in the striatum and hippocampus.41 This increase negatively correlated with the expression of the NMDAR GluN1 subunit, a subunit necessary for the assembly of functional NMDAR. These findings suggest that the age-dependent accumulation of α-syn monomers and oligomers contributes to the reduction in surface NMDAR expression in selective brain regions. An additional mechanism by which α-syn might induce synaptic dysfunction at the postsynaptic level is the internalization of specific NMDAR subunits. It has been reported that α-syn oligomers from the plasma of PD patients promote GluN1 internalization in cultured dopaminergic neurons. Thus, reducing this subunit in the neuronal surface impairs NMDAR function and hampers synaptic function and plasticity.42
A recent study investigated the role of rabphilin-3A (Rph3A) in the striatal synaptopathy induced by α-syn PFF.43 Rph3A is a pre- and postsynaptic protein that can interact directly with α-syn and is involved in stabilizing dendritic spines and the synaptic retention of NMDARs.44,45In vivo injection of intrastriatal α-syn-PFF in mice induces an early loss of striatal synapses associated with decreased levels of Rph3A and impairs Rph3A interaction with NMDAR. Pharmacological targeting of Rph3A expression in the striatum or interfering with the Rph3A/α-syn complex prevented the dendritic spine loss and early motor alterations induced by PFF. Interestingly, these interventions also prevented α-syn-induced synaptic loss in primary hippocampal neurons. Thus, therapeutic strategies restoring Rph3A synaptic functions might slow the synaptic dysfunction induced by α-syn aggregates.
Post-translational changes to α-syn, such as glycation, might also influence its detrimental postsynaptic effects. According to the hypothesis that type-2 diabetes mellitus is a risk factor for PD,46 the effect of a glycating agent has been investigated in transgenic mice overexpressing cerebral α-syn.47 Glycation potentiates motor, cognitive and olfactory dysfunctions in α-syn transgenic mice. In these experiments, α-syn selectively accumulated in the midbrain, striatum and prefrontal cortex. Also, glutamate-associated proteins such as NMDAR, AMPAR, glutaminase, vesicular glutamate transporter and excitatory amino acid transporter type 1 in the midbrain were increased. They suggest that glycation accelerates PD-like sensorimotor and cognitive alterations, and the increase of glutamatergic signalling may underly these events.
Interactions between tau and α-syn in the regulation of synaptic activity are a topic of increased interest explored in different studies in A53T mutant α-syn transgenic models and PFF models in combination with tau knockout or alone.48-50 At the synaptic level, tau-dependent, postsynaptic deficits caused by A53T mutant α-syn have been described.51 Increased α-syn expression reduced spontaneous synaptic release. Postsynaptic dysfunctions revealed by decreased miniature postsynaptic current amplitude and decreased AMPAR to NMDAR current ratio were detected. From the mechanistic point of view, these postsynaptic dysfunctions require glycogen synthase kinase 3β-mediated tau phosphorylation, tau mislocalization to dendritic spines and calcineurin-dependent AMPAR internalization.
Among the various factors that interact with α-syn oligomers, the prion protein (PrPC) has been proposed as a mediator of detrimental α-syn. The crosstalk between α-syn oligomers and PrPC has been investigated using PrPC-knockout hippocampal neurons.52 The PrPC deletion prevents the impairment of hippocampal long-term potentiation (LTP) and cognitive deficits induced by α-syn oligomers. Interestingly, α-syn oligomers form a complex with PrPC that triggers the phosphorylation of Fyn kinase via metabotropic glutamate receptor 5 (mGluR5). This molecular complex activates NMDAR and alters calcium homeostasis. Blockade of mGluR5-evoked phosphorylation of NMDAR in α-syn transgenic mice rescued synaptic and cognitive deficits, supporting the idea that this receptor-mediated mechanism is a key factor for α-syn-induced synaptopathy. More recently, this view was questioned,53 since no difference was found in the effects of α-syn oligomers in control animals and PrPC- knockout mice assessed using behavioural and morphological analyses. Thus, the hypothesis that PrPC-related mechanisms play a major role in the initiation and spread of PD requires further investigation.
Not only neurons but also astrocytes might be affected by the synaptopathy induced by α-syn oligomers. In line with this idea, an interesting study has shown that α-syn oligomers induce the calcium-dependent release of glutamate from astrocytes and that mice overexpressing α-syn show increased glutamate release in vivo.54 This extracellular glutamate activates glutamate receptors, including extrasynaptic NMDARs on neurons recorded from cultures and hippocampal slices of α-syn-overexpressing mice. Patch-clamp recordings from outside-out patches showed that α-syn oligomers could directly activate extrasynaptic NMDARs. The oligomers also induced synaptic spine loss. Similar results were reported in human neocortical neurons derived from iPSCs exposed to α-syn oligomers. Nitrosynapsin, a putative inhibitor of extrasynaptic NMDAR, inhibited these detrimental effects.
Taken all together, a growing body of evidence shows how α-syn impacts postsynaptic activity, mainly modulating glutamatergic neurotransmission. As for presynaptic alteration, also at a postsynaptic level, functional changes precede overt neurodegeneration. As a consequence, striatal synaptic plasticity alterations might be early manifestations of a synucleinopathy, even in the absence of other pathological changes.
Striatal synaptic plasticity
Since striatal synaptic plasticity has been considered a correlate of motor learning, the potential effects of misfolded α-syn on this physiological phenomenon are of major importance for understanding its role in motor deficits.
In the striatum, the bursting activity of nigral DA terminals promotes dendritic spine enlargement in a time window of a few seconds after paired pre- and postsynaptic spiking. This phenomenon is the basis for morphological plasticity and is also implicated in synaptic LTP.55 LTP requires a chain of events, including presynaptic activation of the glutamatergic cortical and thalamic terminal neurons, the release of excitatory amino acids onto spiny projection neurons (SPNs) and subsequent activation of postsynaptic glutamate receptors, leading to membrane depolarization and a rise in intracellular calcium. In conjunction with this sequence of events, DA released from nigrostriatal terminals activates postsynaptic D1 DA receptors, leading to protein kinase A activation and intracellular cAMP production. But how does α-syn interfere with this complex chain of events?
Our group has investigated the possibility that α-syn, by interacting at pre-and postsynaptic levels with these mechanisms, might interfere with striatal plasticity and motor learning, producing early motor and behavioural parkinsonian signs. In an initial study, we investigated striatal synaptic plasticity ex vivo.13 We found that the initial acquisition of motor learning is induced by the activation of the DA active transporter (DAT) and is mediated by a D1-dependent LTP in SPNs. Viral induced-overexpression of human α-syn in substantia nigra reduced striatal DAT levels, impaired motor learning and prevented learning-induced LTP before the appearance of dopaminergic neuronal loss. This striatal mechanism of cellular memory during the acquisition of a skill is a novel form of exercise-induced synaptic plasticity. Interestingly, it requires concomitant molecular changes at both pre- and postsynaptic levels, and it is disrupted in the early stage of synucleinopathies.13
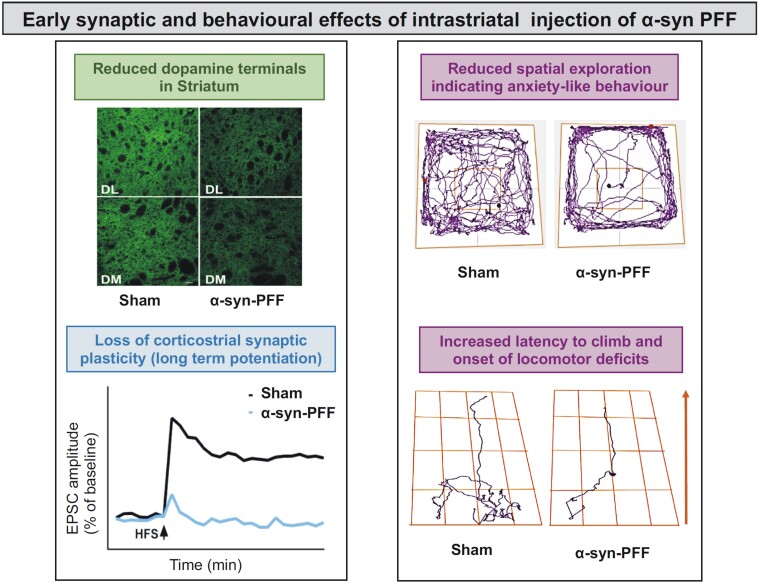
More recently, we observed that intrastriatal injection of α-syn PFF selectively alters the firing rate of dopaminergic neurons in the SNpc, probably diffusing retrogradely.16 Interestingly, the discharge of the GABAergic substantia nigra pars reticulata neurons is unchanged, suggesting a neuronal type-specific vulnerability to α-syn. This α-syn-induced dysregulation of nigrostriatal function was coupled to a time-dependent loss of striatal LTP and long-term depression (LTD) measured on SPNs, and mild behavioural and motor deficits. On the other hand, the spontaneous glutamatergic activity was increased with a presynaptic mechanism. PFF-injected animals showed anxiety-like behaviour and hypokinesia. These changes in neuronal function in the SNpc and striatum, as well as the behavioural dysfunctions, were observed before overt neuronal death occurred, suggesting the appearance of a network dysfunction before neurodegeneration16 (Fig. 4).
Figure 4.
Early synaptic and behavioural effects of intrastriatal injection of α-syn PFF in a rat model. Top left: The reduction in the number of dopaminergic terminals in the dorsolateral (DL) and dorsomedial (DM) striatal regions following α-syn-PFF injection is shown. Bottom left: The loss of corticostriatal long-term potentiation in the spiny projection neurons (SPNs) under the same experimental conditions is represented. The right panel shows how α-syn-PFF intrastriatal injections produce behavioural and motor defects. Top right: Representative track plots of the animal’s exploratory activity in the open field arena show a marked reduction of motor activity and poor exploration in α-syn-PFF-injected rats compared to controls, suggesting the emergence of anxiety-like behaviours. Bottom right: Representative track plots of the animal’s movements in the grid walking setting show a reduced activity that results in an increased latency to climb and a higher immobility time in α-syn-PFF-injected rats compared to the sham-operated rats, suggesting the onset of locomotor deficits (modified from Tozzi et al.16). EPSC = evoked excitatory postsynaptic current; PFF = preformed fibril.
Recent findings obtained by using both in vivo and in vitro models of synucleinopathies show that aberrant α-syn differentially regulates NMDARs function in striatal neurons, according to the receptor subunit expression in the specific cell type. Moreover, α-syn-mediated toxicity at the glutamatergic postsynaptic compartment depends on the structural biophysical characteristics of the protein aggregates and of the neuronal subtype vulnerability. Combining electrophysiological, optogenetic, immunofluorescence, molecular and behavioural analyses, we found that α-syn reduces postsynaptic NMDAR-mediated synaptic currents and impairs corticostriatal LTP of SPNs of both direct (D1-positive) and indirect (D2-positive) pathways.56 Intrastriatal injections of either oligomeric α-syn or PFF produced distinct deficits in visuospatial learning associated with reduced function of the GluN2A NMDAR subunit, indicating that this protein selectively targets this subunit both in vitro and ex vivo (Fig. 3). More specifically, rats injected with oligomeric α-syn showed altered performances in both object displacement and novelty recognition, while PFF-injected animals were partially affected, showing a deficits only in the displacement sessions.
Cholinergic interneurons (ChIs) provide extensive local innervation, which is key in the modulation of striatal microcircuits.57,58 The functional properties of these interneurons influence the activity and plasticity of SPNs, as well as those of other interneurons.59 ChIs play a critical role in striatal activity, underlying action selection and reward in both physiological and pathological conditions.60,61 However, in contrast to the extensive information on the effect of α-syn on the nigrostriatal network, little is known about the role of this protein in affecting the synaptic activity and plasticity of ChIs. We found that overexpression of truncated or wild-type human α-syn in the striatum causes partial reduction of striatal DA levels and selectively blocks the induction of LTP in striatal ChIs, producing early memory and motor alterations.62 These effects depend on α-syn modulation of the GluN2D-NMDAR, a subtype selectively expressed in ChIs (Fig. 3). Acute in vitro application of human α-syn oligomers was also able to block LTP, mimicking the synaptic effects observed ex vivo in PD models. These findings suggest that striatal cholinergic alteration induced by a direct interaction between α-syn and GluN2D-NMDAR represents an early synaptic change in PD.
Taken together, these results support a hypothesis according to which α-syn differentially alters the function of specific NMDARs in different neuronal subtypes. Thus, in addition to the well-recognized brain area vulnerability, emerges the hypothesis that specific features of postsynaptic receptors dictate the synaptic vulnerability of distinct neuronal populations.
Vulnerability of neuronal networks to synuclein spreading
Misfolded α-syn might spread in the brain following anatomically connected networks, and the appearance of clinical signs is seemingly related to this type of spreading.63,64 Recent findings also suggest that α-syn aggregates correspond to different conformational strains of α-syn that can spread in different cell types, causing distinct clinical features.65,66
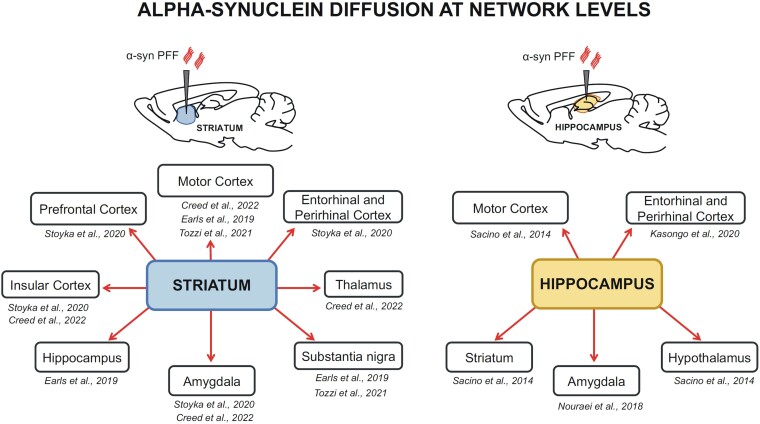
Taking advantage of mouse models, two recent studies explored the spatiotemporal pattern of the spread and transmission of α-syn aggregates. They used quantitative pathology mapping in the mouse brain combined with network modelling.67,68 According to these studies, patterns of α-syn pathology are best predicted by a network model based on two key determinants, such as anatomical connectivity and endogenous α-syn expression. In line with this hypothesis, in the PFF rodent model, intracerebral injections of α-syn-PFF into the striatum (Fig. 5) resulted in increased levels of phosphorylated α-syn at Ser129 (p-α-syn), one of the neuropathological hallmarks of PD, in several brain regions, such as the prefrontal cortex, entorhinal and perirhinal cortex,69 insular cortex,69,70 thalamus70 and hippocampus.71 Evidence of a retrograde diffusion is also provided by the presence of cells immunolabelled with p-α-syn antibodies in the motor cortex, in SNpc,15,68 and the amygdala.69,70 Alpha-syn PFF injections into the hippocampus (Fig. 5) are also able to spread beyond the initial site towards other cognitively-relevant areas, to reach a diffusion of p-α-syn-positive inclusions in the entorhinal and perirhinal cortex,72 amygdala and other limbic areas73 and areas involved in the control of voluntary movements, such as the striatum and the motor cortex.74
Figure 5.
Misfolded α-syn diffusion after in vivo intracerebral injection of PFF in rodent PD models. Schematic representations of the brain regions containing pathological inclusions of α-syn after intracerebral injections of aggregates in the striatum (left) or the hippocampus (right). References to the morphological studies relevant to the brain areas analysed are also reported.62,69-74
Although the factors regulating the spreading of α-syn are still a matter of debate, other critical factors contributing to cell vulnerability must be considered. In fact, not all brain regions or neurons within connected circuits develop α-syn-related pathology. Thus, it has also been proposed that cell-autonomous factors modulate the progression of pathology. Possible cell-autonomous factors are high levels of α-syn, basal oxidative stress, pacemaker activity and elevated cytosolic calcium levels. This poor capacity to degrade misfolding proteins might also be caused by the large axonal arborization of neurons (i.e. dopaminergic cells) and the high rate of mitochondrial turnover.
According to these considerations, a mixed model of cell-autonomous factors and trans-synaptic spread has been proposed to explain the progression of pathology.75 In line with this hypothesis, an experimental study used integrative omics, biochemical and imaging approaches to investigate the molecular events associated with the different stages of LB formation. It demonstrated that LB formation involves a complex interplay between α-syn fibrillization and post-translational modifications, as well as interactions between α-syn and membranous organelles, including mitochondria, the autophagosome and endolysosome.76
In addition to these models, which explain how α-syn spreads in the brain to cause pathology and clinical signs, we should consider that neurodegeneration is preceded by α-syn-induced synaptic impairment in the very early phases of PD. This early synaptic dysfunction might cause precocious network disruption, which is responsible for initial motor and behavioural signs. According to this hypothesis, altered synaptic transmission and loss of physiological synaptic plasticity, as well as changes in firing frequency and patterns in the early phases of the disease, have been detected in various brain areas in animal models.
Most studies presented and discussed to date have focused on α-syn-induced synaptic dysfunction in the basal ganglia. However, two new experimental observations investigating the amygdala, a brain structure critically important for fear and emotions,77,78 as well as to cognitive and behavioural changes in PD,79 add further evidence to the hypothesis of an involvement of other brain networks not directly related to motor control in the non-motor symptoms described in prodromal PD. In the first study, injections of PFF into the striatum caused the formation of α-syn inclusions in the cortex and amygdala. These inclusions primarily localized to excitatory neurons, suggesting the possibility that glutamate-dependent plasticity might be impaired. Interestingly, although no significant loss of neurons was observed in the amygdala or cortex, animals showed functional deficits associated with the prefrontal cortex and amygdala function, such as social dominance behaviour and fear conditioning.69 The second study, using confocal microscopy and slice electrophysiology, along with α-syn knockout mice and PFF, found that glutamatergic cortico-amygdala inputs are selectively affected by α-syn aggregation and consequent synaptic dysfunction.80 These observations, taken together, suggest that α-syn impairs cortical and amygdala function in regions critically important for complex cognitive and behavioural features of PD in the absence of cell loss.
Outcomes of clinical trials: learning from preclinical studies
As extensively discussed, abnormal conformations of α-syn determine deficits in neurotransmission, leading to early clinical manifestations in PD. Therefore, several studies in animal models and patients have tested pharmacological treatments targeting α-syn. The three major strategies are represented by active immunization, passive immunization and aggregation inhibitors.
Concerning active immunization, two peptide vaccines were found to induce a clear immune response.81,82 However, at present, no further information about the clinical efficacy has been released. Recently, another peptide-based vaccine has been tested in healthy controls and early PD patients (NCT04075318), and, also in this case, no outcome has yet been published. With regard to passive immunization, although encouraging preclinical findings were published,83,84 clinical trials in PD patients have failed to meet their primary end points.5,6 Finally, oligomer modulators represent an additional line of investigation, which may potentially provide interesting findings. Among them, an aggregation inhibitor, which was found to reduce the formation of α-syn aggregates and inhibit the formation of oligomers in preclinical studies,85,86 is now being tested in phase 2 clinical trials in early PD patients (NCT04658186). Unfortunately, efficacy outcomes will be released only in the coming years.
The first clinical trials targeting α-syn with the above-reported strategies have not been successful yet. These unmet expectations generated a debate on whether proteinopenia has more relevance than proteinopathy in the pathophysiology of neurodegenerative disorders characterized by protein misfolding and aggregation.87 Multiple reasons can be hypothesized to explain these results. First of all, the treatments targeting α-syn tested in the above reported clinical trials may interfere with the physiological functions of α-syn, such as the regulation of neurotransmitter release and structural conformation of the presynaptic terminals. Therefore, this detrimental effect may overcome the beneficial action on abnormal α-syn and its toxic effects. However, molecules able to distinguish soluble forms of α-syn from pro-aggregation forms, such as the newest oligomer modulators,85 could overcome this issue. Another aspect to be considered is that, according to the evidence represented in this review, synaptic dysfunctions induced by abnormal α-syn also occur before overt neuronal death and clear motor dysfunction. Nevertheless, most trials recruit patients who already manifest parkinsonism and significant nigro-striatal dopaminergic denervation. It is therefore possible that α-syn-induced synaptic dysfunction is already irreversible at this stage. Trials designed to include very early stage patients or even prodromal patients, defined by research criteria, could partially avoid this bias. Furthermore, trial design could be hampered by the clinical heterogeneity of PD patients, in which specific distributions of synaptic and network dysfunctions may differentially affect the response to therapies targeting α-syn. To address this issue, a deep multimodal characterization of patients should be considered, including standardized clinical evaluation, the use of fluid biomarkers and technology-based approaches to monitor motor and possibly non-motor symptoms and responses to therapies (i.e. wearable devices). Finally, a strict definition of inclusion and exclusion criteria would be crucial.
Conclusions and future directions
Alpha-syn-induced synaptic, plastic and network alterations begin early in the natural history of PD and might affect different brain areas, eventually causing distinct clinical signs, from cognitive and psychiatric symptoms to different motor manifestations. However, these synaptic dysfunctions might also persist in the degenerative phase, intermingled with neuronal loss, and contribute to shaping diverse parkinsonian clinical manifestations.
We have new animal models and novel molecular and neurophysiological techniques to investigate the physiological and detrimental roles of α-syn in specific neuronal subtypes. These resources have enormous potential for understanding disease mechanisms and drug discovery. However, their possible translational application to human disease requires a careful sequence of steps. These new experimental approaches can now identify early phases of the disease in some animal models, but we are aware that identifying a prodromal phase in PD patients is still challenging.
In conclusion, we have begun to understand that therapeutic targeting of α-syn in PD patients requires caution, since this protein exerts important physiological synaptic functions. Moreover, the interactions of α-syn with other molecules may induce synergistic detrimental effects; thus, targeting only α-syn might not be enough. Combined therapies should be considered in the future.
Search strategy and selection criteria
We searched PubMed between July and September 2022 for articles published in English, mainly from 2018 to September 2022. We further examined the reference lists from relevant articles. A combination of keywords related to α-syn, synaptic plasticity and PD was used: ‘alpha-synuclein’, ‘neurodegeneration’, ‘Lewy bodies’, ‘Parkinson’s disease’, ‘striatal dopaminergic transmission’, ‘synaptopathy’, ‘synaptic plasticity’, ‘presynaptic’, ‘postsynaptic, ‘animal models’, ‘mechanisms’. We then selected the most relevant papers with particular attention to studies dealing with the earliest pathological effects of α-syn. A few older seminal studies were included for their importance. The final reference list was generated on the basis of relevance to the topics covered in this review.
Contributor Information
Paolo Calabresi, Sezione di Neurologia, Dipartimento di Neuroscienze, Facoltà di Medicina e Chirurgia, Università Cattolica del Sacro Cuore, Rome, 00168, Italy; Neurologia, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, 00168, Italy.
Giulia Di Lazzaro, Neurologia, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, 00168, Italy.
Gioia Marino, Sezione di Neurologia, Dipartimento di Neuroscienze, Facoltà di Medicina e Chirurgia, Università Cattolica del Sacro Cuore, Rome, 00168, Italy.
Federica Campanelli, Sezione di Neurologia, Dipartimento di Neuroscienze, Facoltà di Medicina e Chirurgia, Università Cattolica del Sacro Cuore, Rome, 00168, Italy.
Veronica Ghiglieri, Neurologia, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, 00168, Italy; Department of Human Sciences and Promotion of the Quality of Life, Università Telematica San Raffaele, Rome, 00166, Italy.
Competing interests
P.C. received/receives research support, speaker honoraria, and support to attend national and international conferences (not related to the present study) from: Abbvie, Bial, Bayer Schering, Biogen-Dompè, Biogen-Idec, Eisai, Lilly, Lundbeck, Lusofarmaco, Merck-Serono, Novartis, Sanofi-Genzyme, Teva, UCB Pharma, Zambon. The other authors report no competing interests.
Funding
This work was supported by grants from the Fresco Parkinson Institute to New York University School of Medicine and The Marlene and Paolo Fresco Institute for Parkinson's and Movement Disorders, which were made possible with support from Marlene and Paolo Fresco (to V.G. and P.C.), by Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR)—PRIN (Bando 2017, Prot. 2017ENN4FY, E.D., F.G., N.B.M., P.C.) and by the Italian Ministry of Health, Ricerca Corrente (to P.C.). This study was partially supported by a NIH grant (NS045962 to P.C. in collaboration with Dr. Papa of Emory University, Atlanta, GA, USA).
References
- 1. Goedert M, Jakes R, Spillantini MG. The synucleinopathies: twenty years on. J Parkinsons Dis. 2017;7(s1):S51–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vázquez-Vélez GE, Zoghbi HY. Parkinson's disease genetics and pathophysiology. Annu Rev Neurosci. 2021;44:87–108. [DOI] [PubMed] [Google Scholar]
- 3. Grosso Jasutkar H, Oh SE, Mouradian MM. Therapeutics in the pipeline targeting. Pharmacol Rev. 2022;74:207–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Teil M, Arotcarena ML, Faggiani E, Laferriere F, Bezard E, Dehay B. Targeting α-synuclein for PD therapeutics: a pursuit on all fronts. Biomolecules. 2020;10:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pagano G, Taylor KI, Anzures-Cabrera J, et al. . Trial of prasinezumab in early-stage Parkinson's disease. N Engl J Med. 2022;387:421–432. [DOI] [PubMed] [Google Scholar]
- 6. Lang AE, Siderowf AD, Macklin EA, et al. . Trial of cinpanemab in early Parkinson's disease. N Engl J Med. 2022;387:408–420. [DOI] [PubMed] [Google Scholar]
- 7. Ezzat K, Sturchio A, Espay AJ. The shift to a proteinopenia paradigm in neurodegeneration. Handb Clin Neurol. 2023;193:23–32. [DOI] [PubMed] [Google Scholar]
- 8. Burré J, Sharma M, Südhof TC. Cell biology and pathophysiology of α-synuclein. Cold Spring Harb Perspect Med. 2018;8:a024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghiglieri V, Calabrese V, Calabresi P. Alpha-synuclein: from early synaptic dysfunction to neurodegeneration. Front Neurol. 2018;9:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dabool L, Juravlev L, Hakim-Mishnaevski K, Kurant E. Modeling Parkinson's disease in adult Drosophila. J Neurosci Methods. 2019;311:89–94. [DOI] [PubMed] [Google Scholar]
- 11. Ko WKD, Bezard E. Experimental animal models of Parkinson's disease: a transition from assessing symptomatology to α-synuclein targeted disease modification. Exp Neurol. 2017;298(Pt B):172–179. [DOI] [PubMed] [Google Scholar]
- 12. Cenci MA, Björklund A. Animal models for preclinical Parkinson's research: an update and critical appraisal. Prog Brain Res. 2020;252:27–59. [DOI] [PubMed] [Google Scholar]
- 13. Giordano N, Iemolo A, Mancini M, et al. . Motor learning and metaplasticity in striatal neurons: relevance for Parkinson's disease. Brain. 2018;141:505–520. [DOI] [PubMed] [Google Scholar]
- 14. Marino G, Calabresi P, Ghiglieri V. Alpha-synuclein and cortico-striatal plasticity in animal models of Parkinson disease. Handb Clin Neurol. 2022;184:153–166. [DOI] [PubMed] [Google Scholar]
- 15. Kulkarni AS, Burns MR, Brundin P, Wesson DW. Linking α-synuclein-induced synaptopathy and neural network dysfunction in early Parkinson's disease. Brain Commun. 2022;4:fcac165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tozzi A, Sciaccaluga M, Loffredo V, et al. . Dopamine-dependent early synaptic and motor dysfunctions induced by α-synuclein in the nigrostriatal circuit. Brain. 2021;144:3477–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mohamed NV, Larroquette F, Beitel LK, Fon EA, Durcan TM. One step into the future: new iPSC tools to advance research in Parkinson's disease and neurological disorders. J Parkinsons Dis. 2019;9:265–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bose A, Petsko GA, Studer L. Induced pluripotent stem cells: a tool for modeling Parkinson's disease. Trends Neurosci. 2022;45:608–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin M, Mackie PM, Shaerzadeh F, et al. . In Parkinson's patient-derived dopamine neurons, the triplication of α-synuclein locus induces distinctive firing pattern by impeding D2 receptor autoinhibition. Acta Neuropathol Commun. 2021;9:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prots I, Grosch J, Brazdis RM, et al. . α-Synuclein oligomers induce early axonal dysfunction in human iPSC-based models of synucleinopathies. Proc Natl Acad Sci U S A. 2018;115:7813–7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stykel MG, Humphries KM, Kamski-Hennekam E, et al. . α-Synuclein mutation impairs processing of endomembrane compartments and promotes exocytosis and seeding of α-synuclein pathology. Cell Rep. 2021;35:109099. [DOI] [PubMed] [Google Scholar]
- 22. Yamada K, Iwatsubo T. Extracellular α-synuclein levels are regulated by neuronal activity. Mol Neurodegener. 2018;13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu Q, Shaikh MA, Meymand ES, et al. . Neuronal activity modulates alpha-synuclein aggregation and spreading in organotypic brain slice cultures and in vivo. Acta Neuropathol. 2020;140:831–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Torre-Muruzabal T, Devoght J, Van den Haute C, Brône B, Van der Perren A, Baekelandt V. Chronic nigral neuromodulation aggravates behavioral deficits and synaptic changes in an α-synuclein based rat model for Parkinson's disease. Acta Neuropathol Commun. 2019;7:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gcwensa NZ, Russell DL, Cowell RM, Volpicelli-Daley LA. Molecular mechanisms underlying synaptic and axon degeneration in Parkinson's disease. Front Cell Neurosci. 2021;15:626128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lautenschläger J, Stephens AD, Fusco G, et al. . C-terminal calcium binding of α-synuclein modulates synaptic vesicle interaction. Nat Commun. 2018;9:712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Runwal G, Edwards RH. The membrane interactions of synuclein: physiology and pathology. Annu Rev Pathol. 2021;16:465–485. [DOI] [PubMed] [Google Scholar]
- 28. Gao V, Briano JA, Komer LE, Burré J. Functional and pathological effects of α-synuclein on synaptic SNARE complexes. J Mol Biol. 2023;435:167714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hannestad JK, Rocha S, Agnarsson B, Zhdanov VP, Wittung-Stafshede P, Höök F. Single-vesicle imaging reveals lipid-selective and stepwise membrane disruption by monomeric α-synuclein. Proc Natl Acad Sci U S A. 2020;117:14178–14186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fanning S, Selkoe D, Dettmer U. Vesicle trafficking and lipid metabolism in synucleinopathy. Acta Neuropathol. 2021;141:491–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Navarro-Paya C, Sanz-Hernandez M, De Simone A. Plasticity of membrane binding by the central region of α-synuclein. Front Mol Biosci. 2022;9:857217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. García-Sanz P, Aerts JMFG, Moratalla R. The role of cholesterol in α-synuclein and Lewy body pathology in GBA1 Parkinson's disease. Mov Disord. 2021;36:1070–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schechter M, Atias M, Abd Elhadi S, Davidi D, Gitler D, Sharon R. α-Synuclein facilitates endocytosis by elevating the steady-state levels of phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2020;295:18076–18090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bridi JC, Bereczki E, Smith SK, et al. . Presynaptic accumulation of α-synuclein causes synaptopathy and progressive neurodegeneration in Drosophila. Brain Commun. 2021;3:fcab049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Somayaji M, Cataldi S, Choi SJ, Edwards RH, Mosharov EV, Sulzer D. A dual role for α-synuclein in facilitation and depression of dopamine release from substantia nigra neurons in vivo. Proc Natl Acad Sci U S A. 2020;117:32701–32710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. González-Rodríguez P, Zampese E, Stout KA, et al. . Disruption of mitochondrial complex I induces progressive parkinsonism. Nature. 2021;599:650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Faustini G, Bono F, Valerio A, Pizzi M, Spano P, Bellucci A. Mitochondria and α-synuclein: friends or foes in the pathogenesis of Parkinson's disease? Genes (Basel). 2017;8:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Merino-Galán L, Jimenez-Urbieta H, Zamarbide M, et al. . Striatal synaptic bioenergetic and autophagic decline in premotor experimental parkinsonism. Brain. 2022;145:2092–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wong YC, Luk K, Purtell K, et al. . Neuronal vulnerability in Parkinson disease: should the focus be on axons and synaptic terminals? Mov Disord. 2019;34:1406–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jagtap S, Potdar C, Yadav R, Pal PK, Datta I. Dopaminergic neurons differentiated from LRRK2 I1371V-induced pluripotent stem cells display a lower yield, α-synuclein pathology, and functional impairment. ACS Chem Neurosci. 2022;13:2632–2645. [DOI] [PubMed] [Google Scholar]
- 41. Yang W, Yu W, Li X, Yu S. Alpha-synuclein differentially reduces surface expression of N-methyl-d-aspartate receptors in the aging human brain. Neurobiol Aging. 2020;90:24–32. [DOI] [PubMed] [Google Scholar]
- 42. Yu W, Yang W, Li X, Yu S. Alpha-synuclein oligomerization increases its effect on promoting NMDA receptor internalization. Int J Clin Exp Pathol. 2019;12:87–100. [PMC free article] [PubMed] [Google Scholar]
- 43. Ferrari E, Scheggia D, Zianni E, et al. . Rabphilin-3A as a novel target to reverse α-synuclein-induced synaptic loss in Parkinson's disease. Pharmacol Res. 2022;183:106375. [DOI] [PubMed] [Google Scholar]
- 44. Franchini L, Stanic J, Barzasi M, et al. . Rabphilin-3A drives structural modifications of dendritic spines induced by long-term potentiation. Cells. 2022;11:1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Italia M, Ferrari E, Diluca M, Gardoni F. NMDA And AMPA receptors at synapses: novel targets for tau and α-synuclein proteinopathies. Biomedicines. 2022;10:1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cheong JLY, de Pablo-Fernandez E, Foltynie T, Noyce AJ. The association between type 2 diabetes mellitus and Parkinson's disease. J Parkinsons Dis. 2020;10:775–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chegão A, Guarda M, Alexandre BM, et al. . Glycation modulates glutamatergic signaling and exacerbates Parkinson's disease-like phenotypes. NPJ Parkinsons Dis. 2022;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Singh B, Covelo A, Martell-Martínez H, et al. . Tau is required for progressive synaptic and memory deficits in a transgenic mouse model of α-synucleinopathy. Acta Neuropathol. 2019;138:551–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pan L, Li C, Meng L, et al. . Tau accelerates α-synuclein aggregation and spreading in Parkinson's disease. Brain. 2022;145:3454–3471. [DOI] [PubMed] [Google Scholar]
- 50. Vermilyea SC, Christensen A, Meints J, et al. . Loss of tau expression attenuates neurodegeneration associated with α-synucleinopathy. Transl Neurodegener. 2022;11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Teravskis PJ, Covelo A, Miller EC, et al. . A53t mutant alpha-synuclein induces tau-dependent postsynaptic impairment independently of neurodegenerative changes. J Neurosci. 2018;38:9754–9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ferreira DG, Temido-Ferreira M, Vicente Miranda H, et al. . α-Synuclein interacts with PrP. Nat Neurosci. 2017;20:1569–1579. [DOI] [PubMed] [Google Scholar]
- 53. La Vitola P, Beeg M, Balducci C, et al. . Cellular prion protein neither binds to alpha-synuclein oligomers nor mediates their detrimental effects. Brain. 2019;142:249–254. [DOI] [PubMed] [Google Scholar]
- 54. Trudler D, Sanz-Blasco S, Eisele YS, et al. . α-Synuclein oligomers induce glutamate release from astrocytes and excessive extrasynaptic NMDAR activity in neurons, thus contributing to synapse loss. J Neurosci. 2021;41:2264–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Urakubo H, Yagishita S, Kasai H, Ishii S. Signaling models for dopamine-dependent temporal contiguity in striatal synaptic plasticity. PLoS Comput Biol. 2020;16:e1008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Durante V, de Iure A, Loffredo V, et al. . Alpha-synuclein targets GluN2A NMDA receptor subunit causing striatal synaptic dysfunction and visuospatial memory alteration. Brain. 2019;142:1365–1385. [DOI] [PubMed] [Google Scholar]
- 57. Poppi LA, Ho-Nguyen KT, Shi A, Daut CT, Tischfield MA. Recurrent implication of striatal cholinergic interneurons in a range of neurodevelopmental, neurodegenerative, and neuropsychiatric disorders. Cells. 2021;10:907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Abudukeyoumu N, Hernandez-Flores T, Garcia-Munoz M, Arbuthnott GW. Cholinergic modulation of striatal microcircuits. Eur J Neurosci. 2019;49:604–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Albaugh DL, Gittis AH. Stressing the importance of cholinergic interneurons in striatal function. Mov Disord. 2022;37:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tanimura A, Pancani T, Lim SAO, et al. . Striatal cholinergic interneurons and Parkinson's disease. Eur J Neurosci. 2018;47:1148–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tubert C, Murer MG. What's wrong with the striatal cholinergic interneurons in Parkinson's disease? Focus on intrinsic excitability. Eur J Neurosci. 2021;53:2100–2116. [DOI] [PubMed] [Google Scholar]
- 62. Tozzi A, de Iure A, Bagetta V, et al. . Alpha-synuclein produces early behavioral alterations via striatal cholinergic synaptic dysfunction by interacting with GluN2D N-methyl-D-aspartate receptor subunit. Biol Psychiatry. 2016;79:402–414. [DOI] [PubMed] [Google Scholar]
- 63. Killinger BA, Kordower JH. Spreading of alpha-synuclein—relevant or epiphenomenon? J Neurochem. 2019;150:605–611. [DOI] [PubMed] [Google Scholar]
- 64. Guo YJ, Xiong H, Chen K, Zou JJ, Lei P. Brain regions susceptible to alpha-synuclein spreading. Mol Psychiatry. 2022;27:758–770. [DOI] [PubMed] [Google Scholar]
- 65. Shahnawaz M, Mukherjee A, Pritzkow S, et al. . Discriminating α-synuclein strains in Parkinson's disease and multiple system atrophy. Nature. 2020;578:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Van der Perren A, Gelders G, Fenyi A, et al. . The structural differences between patient-derived α-synuclein strains dictate characteristics of Parkinson's disease, multiple system atrophy and dementia with Lewy bodies. Acta Neuropathol. 2020;139:977–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Henderson MX, Cornblath EJ, Darwich A, et al. . Spread of α-synuclein pathology through the brain connectome is modulated by selective vulnerability and predicted by network analysis. Nat Neurosci. 2019;22:1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rahayel S, Mišić B, Zheng YQ, et al. . Differentially targeted seeding reveals unique pathological alpha-synuclein propagation patterns. Brain. 2022;145:1743–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stoyka LE, Arrant AE, Thrasher DR, et al. . Behavioral defects associated with amygdala and cortical dysfunction in mice with seeded α-synuclein inclusions. Neurobiol Dis. 2020;134:104708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Creed RB, Memon AA, Komaragiri SP, Barodia SK, Goldberg MS. Analysis of hemisphere-dependent effects of unilateral intrastriatal injection of α-synuclein pre-formed fibrils on mitochondrial protein levels, dynamics, and function. Acta Neuropathol Commun. 2022;10:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Earls RH, Menees KB, Chung J, et al. . Intrastriatal injection of preformed alpha-synuclein fibrils alters central and peripheral immune cell profiles in non-transgenic mice. J Neuroinflammation. 2019;16:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kasongo DW, de Leo G, Vicario N, Leanza G, Legname G. Chronic α-synuclein accumulation in rat hippocampus induces Lewy bodies formation and specific cognitive impairments. eNeuro. 2020;7:ENEURO.0009-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nouraei N, Mason DM, Miner KM, et al. . Critical appraisal of pathology transmission in the α-synuclein fibril model of Lewy body disorders. Exp Neurol. 2018;299(Pt A):172–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sacino AN, Brooks M, McKinney AB, et al. . Brain injection of α-synuclein induces multiple proteinopathies, gliosis, and a neuronal injury marker. J Neurosci. 2014;34:12368–12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Henderson MX, Henrich MT, Geibl FF, Oertel WH, Brundin P, Surmeier DJ. The roles of connectivity and neuronal phenotype in determining the pattern of α-synuclein pathology in Parkinson's disease. Neurobiol Dis. 2022;168:105687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mahul-Mellier AL, Burtscher J, Maharjan N, et al. . The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc Natl Acad Sci U S A. 2020;117:4971–4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Inman CS, Bijanki KR, Bass DI, Gross RE, Hamann S, Willie JT. Human amygdala stimulation effects on emotion physiology and emotional experience. Neuropsychologia. 2020;145:106722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Puccetti NA, Schaefer SM, van Reekum CM, et al. . Linking amygdala persistence to real-world emotional experience and psychological well-being. J Neurosci. 2021;41:3721–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Carey G, Görmezoğlu M, de Jong JJA, et al. . Neuroimaging of anxiety in Parkinson's disease: a systematic review. Mov Disord. 2021;36:327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chen L, Nagaraja C, Daniels S, et al. . Synaptic location is a determinant of the detrimental effects of α-synuclein pathology to glutamatergic transmission in the basolateral amygdala. Elife. 2022;11:e78055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Poewe W, Volc D, Seppi K, et al. . Safety and tolerability of active immunotherapy targeting α-synuclein with PD03A in patients with early Parkinson's disease: a randomized, placebo-controlled, phase 1 study. J Parkinsons Dis. 2021;11:1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Volc D, Poewe W, Kutzelnigg A, et al. . Safety and immunogenicity of the α-synuclein active immunotherapeutic PD01A in patients with Parkinson's disease: a randomised, single-blinded, phase 1 trial. Lancet Neurol. 2020;19:591–600. [DOI] [PubMed] [Google Scholar]
- 83. Bae EJ, Lee HJ, Rockenstein E, et al. . Antibody-aided clearance of extracellular α-synuclein prevents cell-to-cell aggregate transmission. J Neurosci. 2012;32:13454–13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Masliah E, Rockenstein E, Mante M, et al. . Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS One. 2011;6:e19338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85. Wagner J, Ryazanov S, Leonov A, et al. . Anle138b: a novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson's disease. Acta Neuropathol. 2013;125:795–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Smit JW, Basile P, Prato MK, et al. . Phase 1/1b studies of UCB0599, an oral inhibitor of α-synuclein misfolding, including a randomized study in Parkinson's disease. Mov Disord. 2022;37:2045–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Espay AJ, Okun MS. Abandoning the proteinopathy paradigm in Parkinson disease. JAMA Neurol. 2023;80:123–124. [DOI] [PubMed] [Google Scholar]