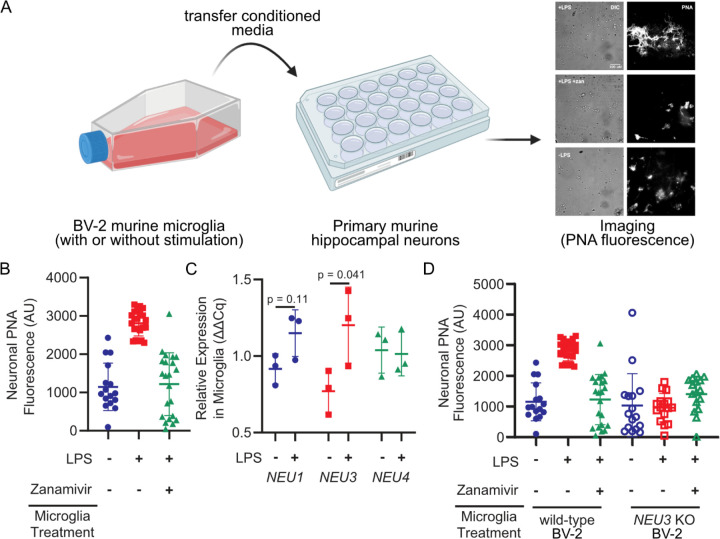
Figure 1. Microglia upregulate and NEU3 and active Neu3 is necessary for secreted sialidase activity.
(A, B) Primary mouse hippocampal neurons were treated with conditioned media from resting or LPS-activated BV-2 microglia in the presence or absence of zanamivir. Representative scheme and images (A) and quantification of fluorescence (B) reveal that LPS-activation causes 3-fold increase in PNA signal compared to resting (+LPS vs. -LPS, p=0.045), an effect abrogated by pharmacological sialidase inhibition (-LPS vs. +LPS +zan, p=0.90; +LPS vs. +LPS +zan, p=0.042). Hypothesis testing performed with hierarchical permutation test, n=3 coverslips/condition, avg. 20 neurons/condition. (C) Quantification of transcript levels of NEU1, NEU3, and NEU4 by qPCR in resting and LPS-activated BV-2 microglia (NEU1, p=0.11; NEU3, p=0.041; NEU4, p=0.90). (D) Neurons were treated with conditioned media from wild-type (WT) or NEU3 knockout (NEU3 KO) BV-2 microglia, with or without deoxy-2,3-anhydroneuraminic acid (DANA), and stained with peanut agglutinin (PNA). Media from activated WT microglia produced a 3-fold increase in desialylation compared to resting (-LPS vs. +LPS, p=0.043; +LPS vs. +LPS+zan, p=0.042) but media from NEU3 KO microglia exhibited no significant change in desialylation in response to LPS or zanamivir (-LPS vs. +LPS, p=0.74; +LPS vs. +LPS+zan, p=0.12). n=3 coverslips/condition, 60 total WT cells, 48 total NEU3 KO cells. Hypothesis tests performed with hierarchical permutation test.