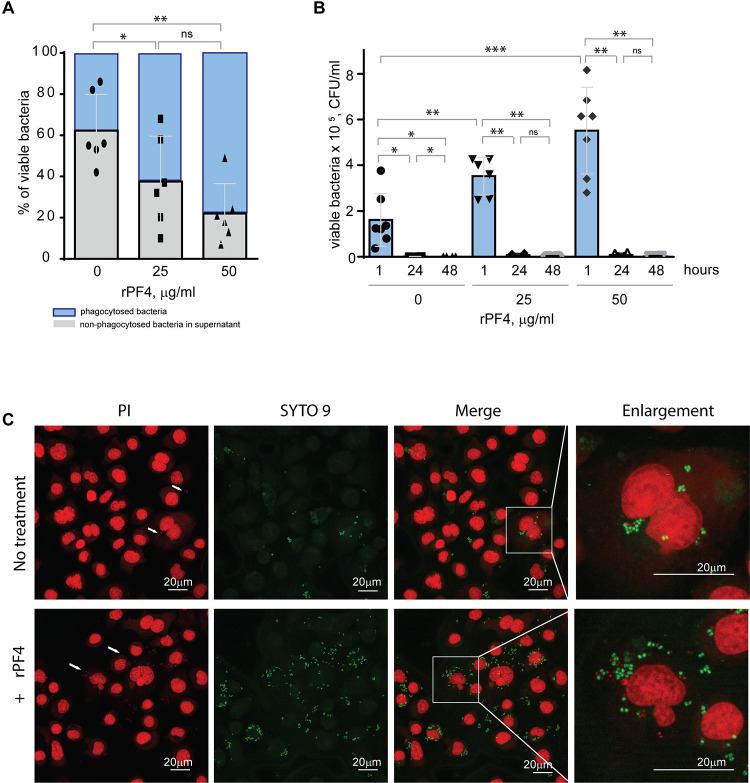
Figure 7. Effect of rPF4 on intracellular bacterial killing.
(A) Adherent IC-21 macrophages (5×105) were incubated with 106 CFU of S. aureus and two concentrations of rPF4 (25 and 50 μg/ml) for 1 h at 37 °C. Aliquots of media containing nonphagocytosed bacteria were plated on LB agar for 16 h at 37 °C to count CFU/ml of viable cells. Data shown are means ± S.D. of six experiments and expressed as a percentage of nonphagocytosed (grey) and phagocytosed bacteria (blue). *p≤ 0.05, **p≤ 0.01, ns, no significant difference. (B) After phagocytosis, cells were washed, and extracellular bacteria potentially remaining on the cell surface were inactivated by treatment with gentamicin for 1 h, after which macrophages were either lysed with 0.2% Triton X-100 immediately or cultured in DMEM for 24 and 48 hours. Aliquots of cell lysates were plated on LB agar plates for 16 h at 37 °C, and CFUs determined. Data are means ± S.D. from six experiments. *p≤ 0.05, **p≤ 0.01, ***p< 0.001, ns, no significant difference. (C) Representative confocal images of macrophages stained with propidium iodide and SYTO 9 to distinguish between live and dead intracellular bacteria. Adherent IC-21 macrophages were incubated with S. aureus treated with 50 μg/ml rPF4 for 1 hour at 37 °C, treated with gentamicin for 1 h, and incubated for an additional 3 h in DMEM. Cells were permeabilized with 0.2% Triton X-100 and stained with a 1:1 mixture of propidium iodide (red) and SYTO 9 (green). Viable S. aureus bacteria are stained green, and dead bacteria are stained red. The scale bars are 20 μm.