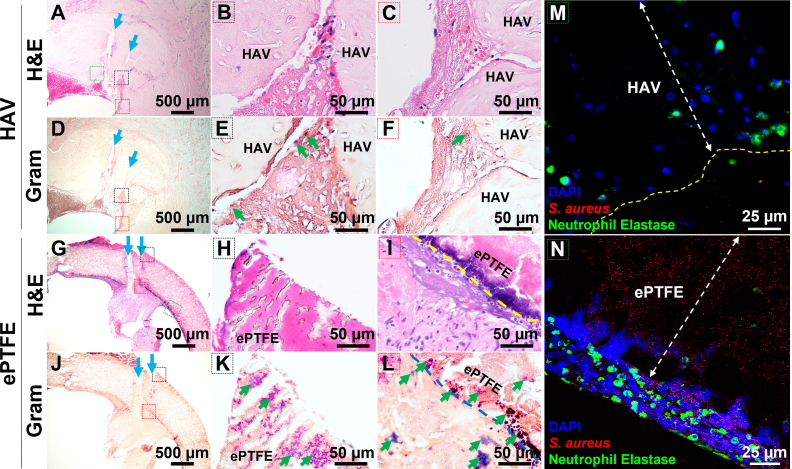
Fig 1.
Representative hematoxylin and eosin (H&E), Gram stain, and immunofluorescence images of explanted human acellular vessels (HAV) and expanded polytetrafluoroethylene (ePTFE) dialysis access conduits, determined to be infected approximately 1 year after implantation in a phase III clinical trial. H&E images show multiple cannulation tracts (blue arrows) from dialysis access through the wall of both the HAV (A-C) and ePTFE graft (G-I). A few clusters of gram-positive bacteria (dark blue) were identified in the thrombus and fibrous tissue but not seen within the wall of the HAV explant (D-F, green arrows). Numerous clusters of bacteria were found inside the wall (K) and within the luminal pannus tissue (L) of the ePTFE graft. The black and red dashed line boxes on the low-magnification images (A, D, G J) correspond with regions shown in color labeled high-magnification H&E and gram stain images. Immunostaining for neutrophils (green) and Staphylococcus aureus bacteria (red) in the HAV (M) and ePTFE (N) explants are shown at high magnification near regions identified with green dashed-line boxes in low-magnification images (A, G). Neutrophils were typically found both outside and inside of the HAV (M), but only along the exterior and not within the contaminated ePTFE graft (N). 4′,6-Diamidino-2-phenylindole (DAPI) stained cell nuclei (blue) as well as diffuse extracellular nuclear material into edge of ePTFE (N) which suggested a loss of neutrophil viability or NETosis at this interface.