Abstract
Introduction
Immune checkpoint inhibitors have revolutionized the treatment of patients with advanced urothelial carcinoma (UC) in the frontline and relapsed settings. Lebanon has one of the highest incidence of UC worldwide, yet no data exists regarding the expression of PD-L1 by Combined Positive Score (CPS) in advanced disease.
Methods
We reviewed all patients treated at our institution for high grade UC, stage pT2 and above, between January 2017 and March 2021. We assessed the expression of PD-L1 by immunohistochemistry using 22C3 clone, and analyzed the association between PD-L1 expression and clinicopathological characteristics. PD-L1 positivity was defined as CPS score ≥ 10.
Results
A total of 101 patients with advanced UC were included, with a median age of 71 years (range, 38 to 96 years); 78% were ever-smokers. Ninety-three of 101 patients (92%) had conventional UC and 43 patients (43%) had positive PD-L1 expression, with 12 patients having CPS of 100. The analysis by molecular subtype showed that patients with maximal CPS of 100 were enriched in “basal” molecular subtype. However, no association was found between PD-L1 expression (positive versus negative) and clinicopathological characteristics.
Conclusion
The positivity of PD-L1 expression as assessed by CPS using the 22C3 clone in our population was almost comparable to the results reported in the occidental literature. Therefore, PD-L1 expression, as a potential predictor of response to immunotherapy, concerns the same percentage of the Lebanese UC patients.
Keywords: Urothelial carcinoma, PD-L1, CPS, 22C3 clone, Bladder cancer
Introduction
The incidence of urothelial carcinoma (UC) in Lebanon is ranked the highest in the Middle East and in the world. In 2018, Lebanon reported the highest age-standardized rate (ASR) for bladder cancer (BC) in men and women, with 25.0 cases per 100,000 individuals [1]. During the last decade, frontline therapies for muscle-invasive or metastatic UC have relied primarily on cisplatin-based chemotherapies. More recently, in the era of immunotherapy, checkpoint inhibitors including anti-PD-1 and anti-PD-L1 agents, have been approved for the treatment of locally advanced or metastatic UC. Atezolizumab and pembrolizumab have been approved by the United States Food and Drug Administration’s (FDA) as a first-line therapy in patients ineligible for platinum-based chemotherapy and whose tumors have positive PD-L1 expression [2, 3]. However, the predictive role of PDL-1 for response to immunotherapy remains uncertain or inconclusive in other settings such as early stages or second-line and maintenance therapy in advanced stages [4].
In order to determine which patients would derive the most benefit from these therapies, the FDA and the European Medicines Agency (EMA) introduced “companion tests” that evaluate the immunohistochemical expression of PD-L1 within tumors cells (TC) and tumor infiltrating immune cells (IC) of the tumor microenvironment (TMI). Until now, the FDA has approved the use of four antibodies (22C3, 28–8, SP263 and SP142) with different methods for evaluating PD-L1 expression [5]. The prevalence of PD-L1 expression as detected by the 22C3 antibody using the CPS (Combined Positive Score) scoring method in locally advanced or metastatic UC in the Lebanese population is yet to be determined. The primary objective of our study is to assess this prevalence among patients diagnosed with muscle invasive or advanced UC in a Lebanese population.
Material and methods
Patient selection
Patients diagnosed with high grade UC, stage pT2 and above, and treated at Hotel-Dieu de France university hospital, with available Formalin-Fixed/Paraffin-Embedded (FFPE) specimens collected between January 2017 and March 2021 were included. Tumor specimens had to contain at least 100 viable tumor cells to ensure tissue adequacy.
Patients’ data was collected from computerized patient files and included age, sex, smoking status, clinical staging, estimated glomerular filtration rate (eGFR) and previous treatment when applicable (Bacillus Calmette-Guerin [BCG] treatment, chemotherapy and/or pelvic radiation therapy).
For all patients, a systematic histologic review was performed in order to confirm the initial diagnosis and determine histopathological criteria including the histologic variant as per the fourth edition of the World Health Organization (WHO) classification of tumors of the urinary system, histologic differentiation, molecular group when available (luminal, basal), histologic staging (pTNM) and group staging as defined by the 8th edition of the American Joint Committee on Cancer (AJCC).
Immunohistochemistry (IHC) with anti-PD-L1 antibodies (22C3 clone)
PD-L1 IHC was performed using the 22C3 pharmDX antibodies on Dako Autostainer Link 48, according to the provider technical manual, on 4-5 µm-thick tissue sections, using DAB detection system. For each case, two slides were performed: one with the PD-L1 antibody and one with a Negative Reagent Control (NRC). Each slide also contained a negative and a positive in-house control. Slides were independently evaluated by two trained pathologists. In case of discrepancy, a consensus was reached, or the mean value was adopted.
Controls were examined first, in order to invalidate any slide with unwanted staining. Slides were examined at × 200 magnification. We took into account any membranous staining in TC and any membranous and/or cytoplasmic staining and IC of TMI. Necrotic and apoptotic TC, plasma cells, neutrophils and fibroblasts were not evaluated. For UC, PD-L1 expression was interpreted using the CPS scoring system with a maximal value of 100:
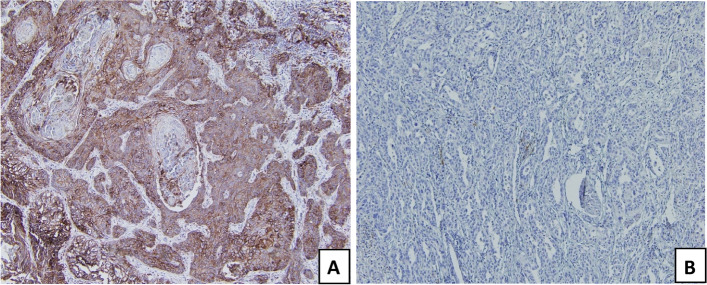
A CPS equal to or greater than 10 was considered positive (Fig. 1) [6].
Fig. 1.
Immunohistochemical stainings showing A positive PD-L1 expression with CPS score of 100 and B negative PD-L1 expression with CPS score of 2 (× 100)
Statistical analysis
Continuous variables were described by medians and ranges while categorical variables were described by effectives (n) and percentages (%). PD-L1 score was analyzed both as a continuous variable (with a score value between 0 and 100) and as a categorical value (negative when inferior to 10 and positive when equal or superior to 10). Chi2 test was conducted to detect any correlation between two categorical variables. Wilcoxon Mann–Whitney test was used to compare continuous variables between two groups. The threshold for statistical significance was set for P-value < 0.05. Statistical analyses were performed using SPSS Statistics version 25.0 (IBM Corporation, New York, USA).
Results
Patient characteristics
A total of 101 patients with muscle invasive or advanced UCs were reviewed. The median age was 71 years (range, 38 to 96 years), 91 patients (90%) were age 60 years and older, and the majority were men (76%). Seventy-nine patients (78%) were either current or former smokers. Among the 101 patients, two had a previous history of occupational exposure to know carcinogens: one worked in the wood industry and the second in the metal industry. Both patients were also smokers. Twenty-five patients (25%) had an altered renal function with an estimated glomerular filtration rate < 50 mL/min. Thirty-nine patients (39%) had metastatic disease defined by involvement of lymph nodes beyond the common iliacs and/or non-lymph-node distant metastases. Twenty-one out of the 39 patients had lymph node metastases, 10 patients had lung metastases, seven patients had bone metastases, and 10 patients had involvement of other organs (Table 1).
Table 1.
Patient characteristics
| Characteristics | N (%) Median [range] |
|---|---|
| Age (years) | 71 [38–96] |
| ≥ 60 years | 91 (90) |
| Male sex | 77 (76) |
| Smoking status | |
| Current smoker | 45 (47) |
| Former smoker | 29 (31) |
| Non smoker | 21 (22) |
| eGFR (mL/min) | 77 [7–149] |
| < 50 mL/min | 25 (25) |
| Stage group | |
| Stage II | 48 (48) |
| Stage III | 14 (14) |
| Stage IV | 39 (39) |
| Prior BCG treatment | 18 (18) |
| Prior chemotherapy | 12 (12) |
| Prior radiation therapy | 1 (1) |
Abbreviations: BCG Bacillus Calmette-Guerin, eGFR Estimated glomerular filtration rate
Tumor characteristics
Diagnosis of UC was established via trans-urethral resection in 52% of cases, surgical resection and cystectomies in 39% of cases, and sampling from metastatic sites in 6% of cases. Three cases were localized at the upper urinary tract and diagnosed following nephrectomy. Pathology examination revealed that 92% of tumors consisted of conventional UC and 8% were of special variants (six cases of poorly differentiated UC, one case of nested UC and one case of lymphoepithelioma-like UC). Forty-one cases presented with one or more differentiations, mainly squamous (25 cases), glandular (11 cases) and micropapillary (11 cases), sarcomatoïd (three cases), microcystic (one case), and independent cells differentiation (one case) (Table 2).
Table 2.
Pathological characteristics of the tumors
| Characteristics | N, (%) |
|---|---|
| Pathology subtype | |
| Conventional urothelial carcinoma | 93 (92) |
| Special variant of urothelial carcinoma | 8 (8) |
| Inflexion/differentiation | |
| Squamous | 25 (25) |
| Glandular | 11 (11) |
| Micropapillary | 11 (11) |
| Sarcomatoid | 3 (3) |
| Independent cells | 1 (1) |
| Microcyctic | 1 (1) |
| Molecular subtype | |
| Basal | 26 (67) |
| Luminal | 11 (28) |
PD-L1 expression
PD-L1 expression was evaluated by CPS score with reported values ranging from 1 to 100 with a median value of 7. Among 101 tumor specimens, 43 (43%) were found to have positive PD-L1 expression whereas 58 out of 101 (57%) had a CPS score inferior to 10 and were therefore classified as negative. Among the eight UC variants, five had a positive PD-L1 expression (63%). Twelve patients had an intense PD-L1 expression with a CPS score of 100. Of those twelve cases, seven had been analyzed for molecular subtype: six cases (86%) were of “basal” molecular subtype and one case (14%) was of “luminal” subtype.
Correlation between PD-L1 expression and clinicopathological characteristics
We further analyzed the association between PD-L1 expression (positive versus negative) and clinical data (sex, smoking status, stage group, site of metastasis, eGFR, prior therapy) and histopathological and molecular characteristics (UC of specific variant, histologic differentiation, molecular group). No statistically significant association was found (Table 3).
Table 3.
Association between PD-L1 expression and clinicopathological characteristics
| PD-L1 expression | P | ||
|---|---|---|---|
|
Negative CPS (< 10%) N = 58 n, (%) |
Positive CPS (≥ 10%) N = 43 n, (%) |
||
| Age | |||
| < 71 years | 22 (44.9) | 18 (52.9) | 0.376 |
| ≥ 71 years | 27 (55.1) | 16 (47.1) | |
| Sex | |||
| Male | 36 (73.5) | 26 (76.5) | 0.803 |
| Female | 13 (26.5) | 8 (23.5) | |
| Smoking status | |||
| Smoker | 37 (78.7) | 26 (83.9) | 0.770 |
| Non-smoker | 10 (21.3) | 5 (16.1) | |
| Stage group | |||
| Stage II | 22 (44.9) | 10 (29.4) | 0.307 |
| Stage III | 6 (12.2) | 7 (20.6) | |
| Stage IV | 21 (42.9) | 17 (50) | |
| Metastatic site | |||
| Lungs | 6 (12.2) | 3 (8.8) | 0.731 |
| Bones | 5 (10.2) | 2 (5.9) | 0.695 |
| Other | 5 (10.2) | 5 (14.7) | 0.733 |
| Previous treatment | |||
| BCG (+) | 11 (68.8) | 7 (58.3) | 0.698 |
| BCG (-) | 5 (31.3) | 5 (41.7) | |
| Chemotherapy (+) | 5 (33.3) | 5 (41.7) | 0.706 |
| Chemotherapy (-) | 10 (66.7) | 7 (58.3) | |
| Histologic type | |||
| Conventional UC | 55 (95) | 38 (88) | 0.28 |
| UC of special variant | 3 (5) | 5 (12) | |
| Inflexion/histologic differentiation | |||
| Squamous | 15 (26) | 10 (23) | 0.803 |
| Glandular | 8 (14) | 3 (7) | 0.187 |
| Micropapillary | 8 (14) | 3 (7) | 0.695 |
| Sarcomatoid | 0 | 3 (7) | 0.165 |
| With signet-ring cells | 0 | 1 (2) | 0.410 |
| Microcystic | 0 | 1 (2) | 0.410 |
| Molecular subtype | |||
| Basal | 14 (64) | 12 (719) | 0.467 |
| Luminal | 7 (32) | 4 (23) | |
Abbreviations: BCG Bacillus Calmette-Guerin, UC Urothelial carcinoma
Discussion
Our study is the first to evaluate the prevalence of PD-L1 expression in muscle-invasive or metastatic UC, using the CPS scoring system, in Lebanon and the Middle-East. In a previous study, Mukherji et al. reported on 54 cases of muscle invasive urothelial carcinoma of conventional type only, evaluated for PD-L1 expression using the 5H1 clone, with a positivity threshold of 5% [7]. Only five tumors were found to be PD-L1-positive (9%). However, the differences in the scoring system, the positivity threshold and the antibody clone used in that study, preclude any direct comparison with our findings.
A positive PD-L1 expression with a CPS score ≥ 10 was found in 43% of our specimens. This was higher than the values described in the study by Faraj et al., using PD-L1 monoclonal antibody 5H1, reporting only 20% of muscle-invasive urothelial carcinoma expressing PD-L1 [8]. When the same CPS scoring method was used, similar levels of PD-L1 expression were observed across different studies. Bellmunt found a CPS score ≥ 10 in 31% of metastatic bladder cancer patients receiving pembrolizumab or chemotherapy as second-line therapy in the Keynote-045 [9]. Powels reported a CPS score ≥ 10 in 47% of patients with metastatic disease treated as first-line in the Keynote-361 [10].
In our population, a higher PDL-1 expression can be expected due to the high prevalence of smoking in 2020 [11], with 67% of smokers reporting water-pipe alone or in addition to cigarette [12]. However, literature review did not reveal a clear correlation between PD-L1 expression in bladder cancer and tobacco consumption, but revealed some correlation between smoking and the effectiveness of immune checkpoint inhibitors when ever-smokers were compared to never-smokers [13].
The discordance in PD-L1 expression in the literature could be explained by the absence of standardized method for evaluation. Reis et al. reported a 30% positivity rate of PD-L1 in conventional UC using the 22C3 clone when accounting only for positive TC. However, when IC were taken into account, the prevalence of PD-L1 rose to 39% in that same population using the same antibody clone [14]. These results are concordant with our findings, considering that 40% of our conventional urothelial carcinoma are PD-L1 expressors.
Previous studies revealed that urothelial carcinomas with special differentiations were more likely to express PD-L1 [14, 15] (p). In our cohort, 63% (five out of eight cases) of our variants had a positive CPS score. However, given the small sample size, no statistically significant correlation was found. In a trial of 195 patients with advanced UC treated with atezolizumab in the second-line setting, a higher prevalence of PD-L1 expression was observed in patients with “basal” molecular subtype compared with their “luminal” counterpart (60% versus 23%, P < 0.001) [16]. In our cohort, despite the small sample size, “basal” molecular subtype was enriched in patients with maximal CPS score. These findings suggest that tumors with “basal” molecular subtype might be more responsive to treatment with immune checkpoint inhibitors.
Conclusion
The positivity of PD-L1 expression as assessed by CPS using the 22C3 clone in our population of patients with advanced UC was almost comparable to the results reported in the occidental literature. Therefore, PD-L1 expression, as a potential predictor of response to immunotherapy, concerns the same percentage of the Lebanese UC patients. Thus, our population deserves “the luxury” to access immunotherapy for urothelial tumors and avoid any cancer treatment disparities [17].
Acknowledgements
Not applicable.
Disclosure
A funding for this research was provided by MSD IDEA GmbH.
Authors’ contributions
JK and VTS designed the study. SN and FGH drafted the initial manuscript. SN, JKh and VTS collected and reviewed the data. FGH conducted the statistical analysis. All authors carefully reviewed and edited the final manuscript.
Funding
This study was supported by MSD.
Availability of data and materials
Data may be shared to qualified researchers upon reasonable request to the corresponding author. No identifying data will be provided.
Declarations
Ethics approval and consent to participate
This research was approved by the institutional review board at Saint Joseph University of Beirut, Lebanon and performed in accordance with the Declaration of Helsinki. All patients signed an informed consent according to institutional guidelines.
Consent for publication
Not applicable.
Competing interests
S.N. received funding from MSD, F.G.H. received funding from MSD, J. Kattan received funding from MSD, and V.T.S. received funding from MSD. J. Khazen has no conflict of interest to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Institute for Cancer Research. Bladder cancer statistics. https://www.wcrf.org/dietandcancer/cancer-trends/bladder-cancer-statistics. Accessed 14 Jan 2022.
- 2.Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–1492. doi: 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 3.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76. doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gevaert T, Cimadamore A, Montironi R, Eckstein M. PD-L1 testing for urothelial carcinoma: interchangeability, reliability and future perspectives. Curr Drug Targets. 2021;22(2):162–170. doi: 10.2174/1389450121666200510015216. [DOI] [PubMed] [Google Scholar]
- 5.Tsao MS, Kerr KM, Kockx M, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint Phase 2 project. J Thorac Oncol. 2018;13(9):1302–1311. doi: 10.1016/j.jtho.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckstein M, Cimadamore A, Hartmann A, et al. PD-L1 assessment in urothelial carcinoma: a practical approach. Ann Transl Med. 2019;7(22):690. doi: 10.21037/atm.2019.10.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukherji D, Jabbour M, Saroufim M, et al. Pd-L1 expression in muscle-invasive bladder cancer cystectomy specimens and lymph node metastasis. Ann Oncol. 2014;25:iv300. doi: 10.1093/annonc/mdu337.55. [DOI] [PubMed] [Google Scholar]
- 8.Faraj SF, Munari E, Guner G, et al. Assessment of tumoral PD-L1 expression and intratumoral CD8+ T cells in urothelial carcinoma. Urology. 2015;85(3):703.e1–6. doi: 10.1016/j.urology.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powles T, Csőszi T, Özgüroğlu M, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(7):931–945. doi: 10.1016/S1470-2045(21)00152-2. [DOI] [PubMed] [Google Scholar]
- 11.World Population Review. Smoking Rates By Country 2020. https://worldpopulationreview.com/countries/smoking-rates-by-country/. Accessed 14 Jan 2022.
- 12.Waked M, Salameh P, Aoun Z. Water-pipe [narguile] smokers in Lebanon: a pilot study. East Mediterr Health J. 2009;15(2):432–442. doi: 10.26719/2009.15.2.432. [DOI] [PubMed] [Google Scholar]
- 13.Lee KWC, Lord SJ, Kasherman L, et al. The impact of smoking on the effectiveness of immune checkpoint inhibitors - a systematic review and meta-analysis. Acta Oncol. 2020;59(1):96–100. doi: 10.1080/0284186X.2019.1670354. [DOI] [PubMed] [Google Scholar]
- 14.Reis H, Serrette R, Posada J, et al. PD-L1 expression in urothelial carcinoma with predominant or pure variant histology: concordance among 3 commonly used and commercially available antibodies. Am J Surg Pathol. 2019;43(7):920–927. doi: 10.1097/PAS.0000000000001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Zhang Q, Shuman L, et al. Evaluation of PD-L1 and other immune markers in bladder urothelial carcinoma stratified by histologic variants and molecular subtypes. Sci Rep. 2020;10(1):1439. doi: 10.1038/s41598-020-58351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrios C, de Lima LG, Yusof MM, Rubagumya F, Rutkowski P, Sengar M. Barriers in access to oncology drugs — a global crisis. Nat Rev Clin Oncol. 2023;20(1):7–15. doi: 10.1038/s41571-022-00700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be shared to qualified researchers upon reasonable request to the corresponding author. No identifying data will be provided.